New method uses a single gene to reveal neuronal circuits from multiple upstream regions
The body functions via an upstream to downstream flow of information mediated by the brain. Input received through upstream areas like the eyes and ears is distributed to its appropriate downstream region by neurons. Following the expression paths of viruses with viral vector infections and observing light-sensitive proteins in a neuronal population with a fluorescence microscope are some ways in which scientists genetically target these neurons to better understand their structure and function in the overall flow of information. "However, these methods alone are limited in their ability to target neurons that receive monosynaptic inputs from two distinct upstream regions, which in turn inhibits our ability to focus on the structure and function of neurons on a finer scale," states Kenji Mizuseki, Professor of the Department of Physiology, Osaka City University Graduate School of Medicine.
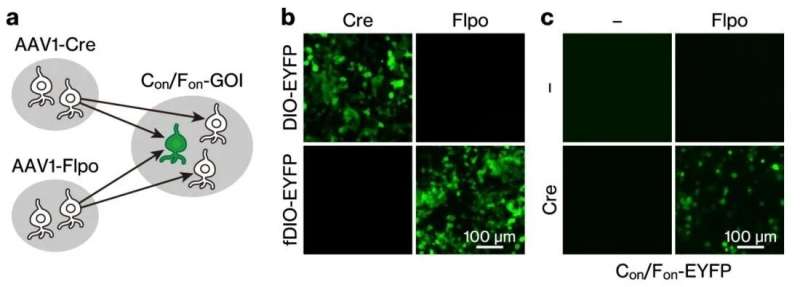
A Communications Biology paper published February 22 details how Professor Mizuseki led a team of researchers to develop a method they call "intersectional, anterograde transsynaptic targeting system," that genetically labels neurons with a single gene of interest (GOI), in this case a gene that encodes an enhanced yellow fluorescent protein. They demonstrate this labeling method in two different mouse brain circuits: the retina/primary visual cortex to the superior colliculus and the bilateral motor cortex to the dorsal striatum.
Viral vectors are tools commonly used to deliver genetic material into cells, allowing one to track the genetic path a virus takes as it combines with other cells. This got the team thinking. They could inject viruses with genes at different upstream areas of the brain that once combined in specially prepared downstream regions, would alter the genetic environment to produce this enhanced yellow fluorescent protein that they could observe with a fluorescence microscope. "This combination of existent technology—adeno-associated virus serotype 1 (AAV1) and intersectional expression system (INTRSECT)", states first author of the study, Takuma Kitanishi, "brings the best from both worlds for a labeling method that could exhibit synaptic specificity."
Using mouse models, the team went to work.
They introduced genetically prepared viruses in the upstream -presynaptic- regions of the right eye and left visual cortex (V1), and in the postsynaptic left superior colliculus (sSC), observing many fluorescence-emitting neurons in the left sSC but not in adjacent regions. "We also observed an absence of fluorescence when either of the upstream viruses was omitted," adds Prof. Mizuseki. To solidify that the team was indeed observing a specific circuit between the retina/V1 and the sSC, they prepared the whole brain by introducing a viral vector that crosses the blood/brain barrier. In addition to fluorescent neurons in the sSC, the team also observed them in the left ventral geniculate nucleus (LGNv). Since this region does not back project to the retina or V1, they hypothesize that the LGNv contains neurons that integrate inputs from the retina and V1. This additional result and subsequent inquiry from testing the robustness of their work is a peak into the potential focus afforded by their new method.
Moving onto the motor cortex (M2), where it is known that the dorsal striatum (DS) receives inputs from M2, the team wanted to find out if DS neurons were receiving inputs from both left and right M2 regions. Applying their method, they saw the fluorescent protein in all types of DS neurons they examined.
"After these experiments," states Prof. Mizuseki, "it is clear our method provides a powerful means for determining the locations, numbers, and cell types of neurons receiving monosynaptic inputs from the two defined upstream regions."
In the future, Prof. Mizuseki expressed interest in expanding this new method by replacing the GOI with one that produces a photosensitive protein that they can then use to excite or suppress neuronal activity with light.
More information: Takuma Kitanishi et al, Intersectional, anterograde transsynaptic targeting of neurons receiving monosynaptic inputs from two upstream regions, Communications Biology (2022). DOI: 10.1038/s42003-022-03096-3