Baby-OSCAR trial evaluates if selective early treatment of PDA reduces death or BPD at 36 weeks in preterm babies

A randomized controlled trial evaluates if selective early treatment of patent ductus arteriosus reduces death or bronchopulmonary dysplasia at 36 weeks in extreme preterm babies. Findings from the study will be presented during the Pediatric Academic Societies (PAS) 2022 Meeting, taking place April 21-25 in Denver.
The management of patent ductus arteriosus continues to be a clinical conundrum in extremely low gestational age newborns. Presence of a patent ductus arteriosus at 72 hours after birth in extremely low gestational age newborns is associated with an increased risk of death and complications of prematurity. Functional echocardiography is increasingly used to assess the haemodynamic impact of patent ductus arteriosus and can be used in the first three days after birth to select babies with a large pre-symptomatic patent ductus arteriosus.
Baby-OSCAR trial is a UK multi-center placebo-controlled masked randomized clinical trial in extremely low gestational age newborns (23+0 to 28+6 weeks gestational age). The objective of the study was to evaluate whether early targeted treatment of a large patent ductus arteriosus (diagnosed by functional echocardiography) with ibuprofen within 72 hours of birth improves short term health outcomes of death or moderate to severe bronchopulmonary dysplasia at 36 weeks post-menstrual age.
Researchers found no evidence of a reduction in death or moderate to severe bronchopulmonary dysplasia with early selective treatment of a large patent ductus arteriosus with ibuprofen within 72 hours of birth in extreme preterm infants.
"The aim of Baby-OSCAR trial was to find out whether or not a large patent ductus arteriosus in extreme preterm babies should be treated with ibuprofen within 72 hours of birth," said Samir Gupta, MD, chief investigator and professor of neonatology at Durham University, UK. "Ibuprofen is a non-steroidal anti-inflammatory drug that is commonly used for pain relief in adults. Patent ductus arteriosus is a condition that is caused by a blood vessel called the ductus arteriosus staying open after a baby's birth. During pregnancy, the ductus arteriosus allows blood from the baby's heart to flow to the mother's placenta to get oxygen, bypassing the baby's lungs. Soon after birth the ductus should close to allow blood to flow to the baby's own lungs to get oxygen. However, in extreme preterm babies the ductus often takes a long time to close on its own and this can lead to a variety of complications. Clinicians are unsure if early treatment should be offered to extreme preterm babies to close the patent ductus arteriosus and reduce the risks of complications, or whether it would be better to wait and see if the ductus will close on its own."
-
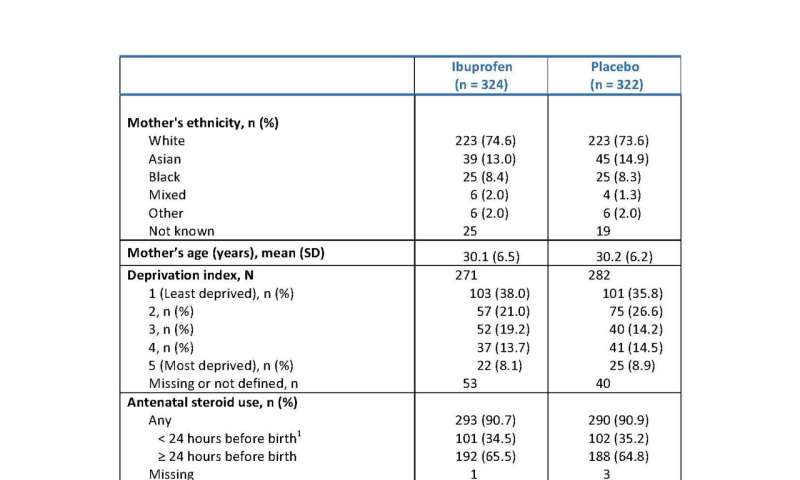
Maternal baseline characteristics. Doses would usually be 24 hours apart, so < 24 hours before birth suggests only one dose was taken. Credit: Department of Engineering, Durham University, United Kingdom -
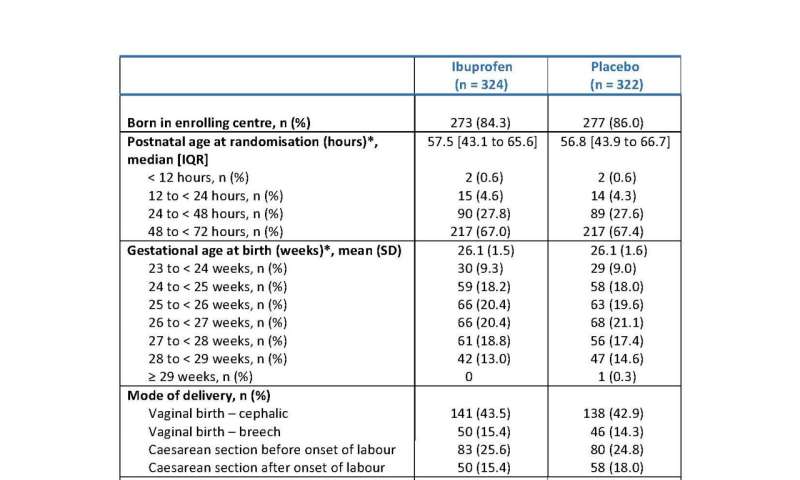
Infant's characteristics at trial entry. 1 Including abnormally implanted placenta. 2 +/- APH. 3 Nasal CPAP, nasal ventilation, humidified high flow nasal cannula therapy, or low flow oxygen ≥ 1.1L/min. 4 In room air, low flow oxygen > 1.1L/min, or ambient oxygen). Credit: Department of Engineering, Durham University, United Kingdom -
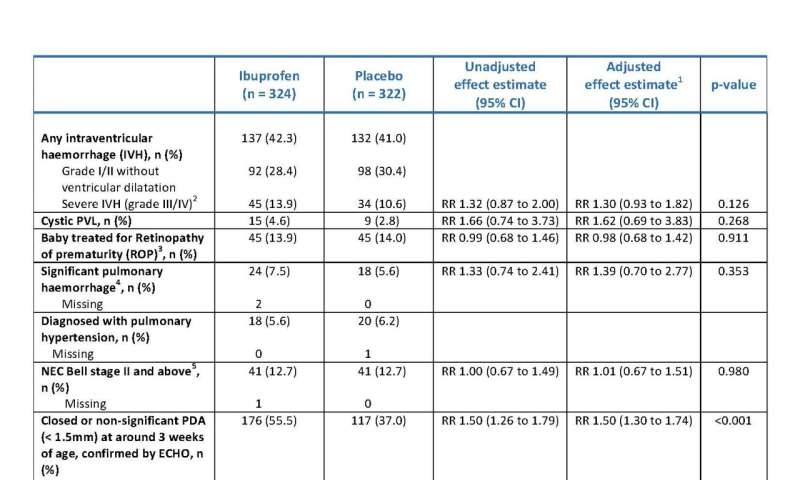
Secondary outcomes (Tested). 1 Adjusted for size of PDA at randomisation, gestational age at birth, age at randomisation, sex, multiple birth, mode of respiratory support at randomisation, receiving inotropes at time of randomisation, and center as a random effect, and clustered by siblings to account for correlation between multiple births. 2 With ventricular dilatation or intraparenchymal abnormality. 3 In at least one eye. 4 Fresh blood in endotracheal tube with increase in respiratory support. 5 Confirmed by radiography and/or histopathology. 6 For descriptive purposes only. Credit: Department of Engineering, Durham University, United Kingdom
Dr. Gupta added that "in the Baby-OSCAR trial, extreme preterm babies were screened in first 72 hours with echocardiography and those with a large patent ductus arteriosus meeting inclusion criteria were randomized to either treatment with i.v. ibuprofen or placebo. About ~4,000 extreme preterm babies were screened in 32 tertiary neonatal intensive care units with echocardiography in the UK, and 653 of them were randomized after parental consent. The results on short term outcomes till discharge are presented. Babies are currently being followed up to two years corrected age for assessment of neurodevelopment and respiratory morbidity and prospective health economic evaluation is conducted."
"The short term outcomes demonstrated no evidence of benefit of early targeted treatment with ibuprofen within 72 hours of birth compared to placebo in extreme preterm babies with a large patent ductus arteriosus on death or moderate/severe bronchopulmonary dysplasia at 36 weeks postmenstrual age. There were no significant differences in other complications of prematurity, however, babies treated with ibuprofen had reduced the risk of an open patent ductus arteriosus at three weeks of age and significantly less need for surgical treatment for a symptomatic patent ductus arteriosus."
More information: Conference: www.pas-meeting.org/