Using smart sensors to ensure vaccine safety
In most methods used today, clinical trials designed to evaluate the safety of a new drug or vaccine employ self-report questionnaires, asking participants how they feel before and after receiving the treatment. A new study from Tel Aviv University enables developers, for the first time in the world, to determine vaccine safety via smart sensors that measure objective physiological parameters. According to the researchers, most clinical trials testing the safety of new vaccines, including COVID-19 vaccines, rely on participants' subjective reports, which can lead to biased results. In contrast, objective physiological data, obtained through sensors attached to the body, is clear and unambiguous.
The study was led by Dr. Yftach Gepner of the Department of Epidemiology and Preventive Medicine at TAU's Sackler Faculty of Medicine, together with Dr. Dan Yamin and Dr. Erez Shmueli from TAU's The Iby and Aladar Fleischman Faculty of Engineering. The paper was published in Communications Medicine, a journal from the Nature portfolio.
The end of an era?
Researchers from Tel Aviv University demonstrated that smart sensors can be used to test new vaccines. The current study was conducted when many Israelis received their second dose of the COVID-19 vaccine. The researchers equipped volunteers with innovative, FDA-approved sensors developed by the Israeli company Biobeat. Attached to their chests, these sensors measured physiological reactions from one day before to three days after receiving the vaccine.
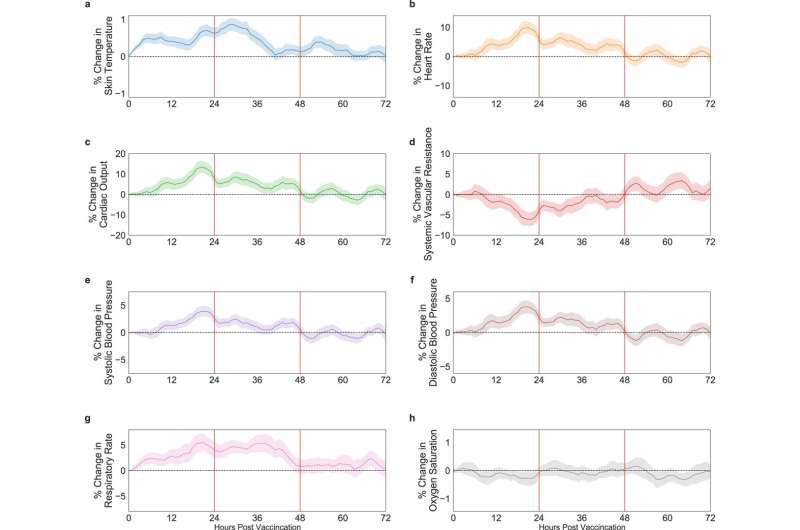
The innovative sensors monitored 13 physiological parameters, such as: heart rate, breathing rate, saturation (blood oxygen levels), heartbeat volume, temperature, cardiac output, and blood pressure. The surprising results:
- A significant discrepancy was found between subjective self-reports about side effects and actual measurements. That is, in nearly all objective measures, significant changes were identified after vaccination, even for subjects who reported having no reaction at all.
- In addition, the study found that side effects escalate over the first 48 hours, and then parameters return to the level measured before vaccination. In other words: a direct assessment of the vaccine's safety identified physiological reactions during the first 48 hours, with levels re-stabilizing afterwards.
"The message from our study is clear," says Dr. Gepner. "In 2022 the time has come to conduct continual, sensitive, objective testing of the safety of new vaccines and therapies. There is no reason to rely on self-reports or wait for the occurrence of rare side effects like myocarditis, an inflammation of the heart muscle, which occurs in one of 10,000 patients. Preliminary signs that predict such conditions can be detected with advanced sensors, identifying normal vs. extreme alterations in physiological parameters and any risk of inflammation. Today, trial participants are invited to the clinic for blood pressure testing, but often their blood pressure rises just because the situation is stressful. Continual monitoring at home solves these problems with simple, convenient, inexpensive, and accurate means. This is the kind of medicine we should strive for in 2022."
More information: Yftach Gepner et al, Utilizing wearable sensors for continuous and highly-sensitive monitoring of reactions to the BNT162b2 mRNA COVID-19 vaccine, Communications Medicine (2022). DOI: 10.1038/s43856-022-00090-y