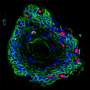
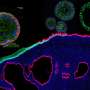
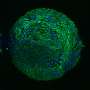
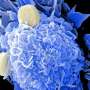
Patient-derived and mouse endo-ectocervical organoid generation
A few stem cells, various growth factors, four to six weeks of time—and, of course, a great deal of expertise—are needed to create a scaled-down but nevertheless lifelike and functional replica of a cervix in the laboratory.
A new publication that has now appeared in the journal Nature Protocols shows how the process works in detail. Dr. Cindrilla Chumduri, head of the research group at the Department of Microbiology at the Julius Maximilians University of Würzburg (JMU), is responsible for this. The infection and cancer biologist has been researching the physiological processes in the cervical tissue for a long time. She is particularly interested in the conditions under which cancer develops there.
"Until recently, science has lacked a system that well reflects the cellular, physiological and functional properties of the different cell types in the cervix," says Chumduri. This, she says, has made it difficult to study normal physiology, disease development and infectious processes.
With the three-dimensional organoids she has developed, she says, "new opportunities are now opening up to study the biology of the cervix, infections and the development of cancer." New applications in personalized medicine, the search for new active substances, interventions on the genome, the modeling of diseases: With the help of organoids, scientists could now put all this into practice much more easily than before.
The cervix has many functions
The cervix is a complicated structure. One of its most important tasks is to enable the passage of sperm into the uterine cavity so that fertilization of the egg can take place. On the other hand, it must protect the female reproductive tract from dangerous invaders such as fungi, viruses and bacteria and from ascending infections. In addition, at the end of a pregnancy, it must be able to dilate significantly so that the fetus can pass through it.
Anatomically, the cervix forms the link between the uterine cavity and the vagina. It consists of the so-called endocervix, which is adjacent to the uterus, and the ectocervix, which protrudes into the vagina. These are lined by different types of cells: While the endocervix has a columnar epithelium, the ectocervix has a multi-layered squamous epithelium. Where the two areas merge, they form a transition zone and are particularly susceptible to infection and tissue formation. For example, most cervical cancers originate there.
Stem cells as starting material
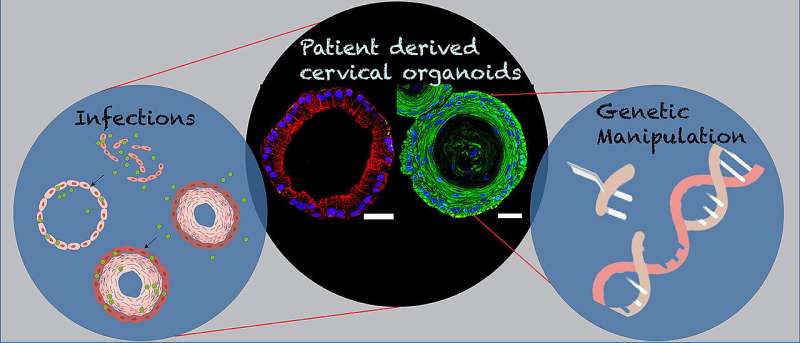
For the development of the 3D organoids of the cervix, Cindrilla Chumduri and her team chose adult epithelial stem cells as starting material. These were taken in biopsies from both the endocervix and the ectocervix. With the help of unique combinations of growth factors as well as different cultivation methods, they were able to recapitulate the natural three-dimensional tissue architecture and composition as well as the functional properties of the original tissue and preserve them over a long period of time.
In more advanced experiments, the scientists also genetically manipulated the stem cells. "We implanted the stem cells with genes from the human papillomavirus HPV, which are responsible for causing cancer to develop," says Chumduri. This could potentially solve a mystery that science has worked on for a long time.
Fatal co-infections
For although it is known that HPV is the driving force behind the majority of cervical cancers, infection with the virus is not synonymous with malignant tissue neoplasm: Current statistics suggest that about 80 percent of all women will experience an HPV infection during their lifetime. Nevertheless, only 1.6 percent of them develop cervical cancer.
It is now suspected that there are other factors that increase the risk of cervical cancer, such as co-infection with other sexually transmitted pathogens, such as the bacterial pathogen Chlamydia trachomatis. The genetically engineered human ectocervical organoids now allow Chumduri and her team to more closely examine the long-term effects of viral infection on the squamous epithelium of the cervix and the contribution of co-infections with other pathogens, such as Chlamydia trachomatis.
Great potential for further progress
"Endocervical and ectocervical organoids are ideal, almost physiological 3D epithelial tissues for studying and modeling cervical biology, host-pathogen interactions and disease development," she is certain. In addition, she says, they are ideal for studying the organ's response to antibiotic-resistant pathogens.
Organoids also make it possible to study the reaction of the cervical epithelium to hormonal changes and their effects on stem cell regeneration, mucus production and innate defense against pathogens. Their long-term cultivability offers a unique opportunity to take a closer look at chronic or repeated infections and their impact on host cells, she said.
In any case, Cindrilla Chumduri is convinced: "Overall, the organoid model of the cervix offers great potential for further advances in the study of the biology of the female reproductive tract."
More information: Rajendra Kumar Gurumurthy et al, Patient-derived and mouse endo-ectocervical organoid generation, genetic manipulation and applications to model infection, Nature Protocols (2022). DOI: 10.1038/s41596-022-00695-6