Managing phage therapy to help save lives
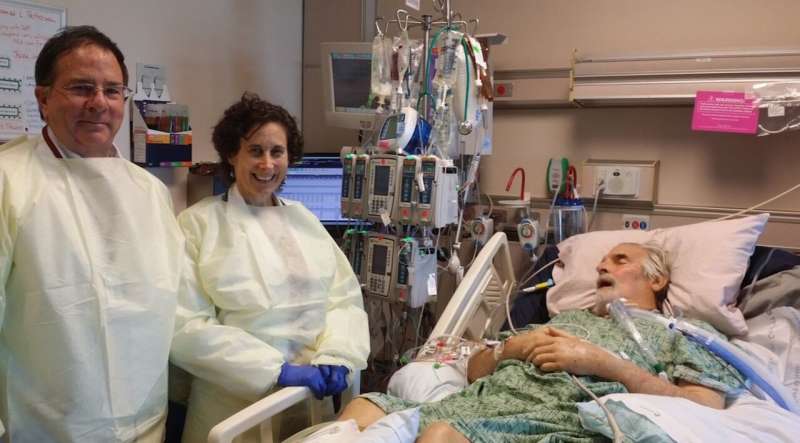
Scientists with the Texas A&M College of Agriculture and Life Sciences were among those providing the biochemical tools needed to help save a man's life through a unique emergency intervention in 2016.
Now those Center for Phage Technology scientists in the Texas A&M Department of Biochemistry and Biophysics, Bryan-College Station, have completed a study about that treatment as well as other opportunities for phage therapy.
Their study, "Comparative genomics of Acinetobacter baumannii and therapeutic bacteriophages from a patient undergoing phage therapy," was published recently in the scientific journal Nature Communications.
The threat of antimicrobial resistance has become a worldwide concern, with the World Health Organization estimating at least 50 million people per year worldwide could die from it by 2050. Center for Phage Technology scientists believe phage therapeutics can be used to fight these resistant bacterial infections.
The premiere case involved phage center scientists working in collaboration with other scientists and physicians at University of California San Diego, UC San Diego, School of Medicine and the U.S. Navy Medical Research Center—Biological Defense Research Directorate. Together, they worked to identify phages and determine a treatment plan for Tom Patterson, a professor of psychiatry at the UC San Diego School of Medicine, who was infected by a deadly pathogen while vacationing in Egypt.
About phages
Bacteriophages, or phages, are viruses that can infect and kill bacteria without having a negative effect on human or animal cells. Phages can be used alone or in combination with antibiotics or other drugs to treat bacterial infections.
"Bacteriophage therapy is an emerging field that many researchers think could yield novel ways to fight antimicrobial-resistant bacteria," said Mei Liu, Ph.D., program director at the Center for Phage Technology and a primary investigator for the study. "At the center, we are interested in the applications of phage therapeutics to fight multidrug-resistant bacterial infections."
She said the center's work is aided by the team's deep knowledge of phage biology, particularly in the areas of phage lysis and phage genomics.
Patterson's predicament
In 2015, while on vacation in Egypt during the Thanksgiving holiday, Patterson began to experience severe abdominal pain, nausea and vomiting. Local doctors diagnosed him with pancreatitis and treated him accordingly, but the treatments didn't work and his condition worsened.
He was later transported to Germany, where doctors found fluid around his pancreas and took cultures from the fluid's contents. The cultures showed he had been infected with a multidrug-resistant strain of Acinetobacter baumannii, an often-deadly pathogen found in hospital settings and in the Middle East. The same pathogen was also identified in many injured U.S. military members returning home after serving in that part of the world.
In Germany, Patterson was treated with a combination of antibiotics, and his condition improved to a degree where he could be airlifted to the intensive care unit at Thornton Hospital in the UC San Diego Health academic health system. There, however, the medical team discovered that the bacteria had become resistant to antibiotics.
A "compassionate use" exemption for phage therapy was requested by Dr. Robert "Chip" Schooley, the UC San Diego physician treating Patterson. He was given rapid approval from the U.S. Food and Drug Administration, FDA, to proceed.
Shortly after the phage treatment began, Patterson awakened from a months-long coma. After a long recovery, his health improved greatly, and he was able to return to life as it was before the infection.
Acinetobacter baumannii and other resistant pathogens
Acinetobacter baumannii is recognized as a significant bacterial pathogen in health care-associated infections. A Centers for Disease Control and Prevention report from 2019 stated that antibiotic-resistant pathogens cause more than 2.8 million infections and more than 35,000 deaths annually in the U.S.
Several characteristics of the pathogen that infected Patterson impacted the treatment regimens and outcomes, said Ry Young, Ph.D., director of the Center for Phage Technology.
Patterson's wife, Steffanie Strathdee, Ph.D., associate dean of global health sciences with UC San Diego School of Medicine and an infectious disease epidemiologist, had contacted Young to seek his help in finding a treatment for her husband once she became aware of Young's extensive work with phages.
Young and his lab team took up the challenge and worked almost nonstop for three months to help find a solution.
"Cases of resistant infections are becoming more prevalent and very few new antibiotics are available, so the use of bacteriophages to treat or control multidrug-resistant infections is being reconsidered as an alternative strategy," Young said. "Phage therapy is actually a very old concept, having been used extensively in the early 20th century during the pre-antibiotic era."
Phage treatment also has been successful in several more recent case studies involving multidrug-resistant strains of P. aeruginosa, Staphylococcus aureus and Escherichia coli bacteria.
"Phages had been sidelined as a potential treatment for bacterial infections when antibiotics came into wide use in the U.S.," Liu said. "But in other areas of the world, particularly where antibiotics were not immediately available, researchers and doctors have continued developing and practicing phage therapy. Now we are seeing more instances of how phage therapy can be used when antibiotics alone are not sufficient to treat bacterial infections."
Lessons from the Patterson case
Jason Gill, Ph.D., professor in the Texas A&M Department of Animal Science and associate director of the Center for Phage Technology, said while the Patterson case and similar case studies treating multidrug-resistant bacteria have been encouraging in terms of clinical outcome, a more in-depth examination of the phage-host interaction during treatment and its implications is needed.
"The recent study showed that resistance to the therapeutic phages emerged early, and the acquisition of new mobile elements by the bacteria can occur during treatment," said Gill, a corresponding author of the study. "It is important to have a thorough genomic analysis of phages prior to phage treatment in order to maximize treatment success and minimize both effort and resources. There is also a need for conventional experimental testing for phage host range and growth characteristics."
Gill also noted the use of well-characterized phages in a phage cocktail can avoid redundancy and significantly save time and effort in phage production and purification. Eight of the nine phages used for treatment in the Patterson case turned out to be closely related, and this knowledge could have been used to streamline the process if the investigators had known this when assembling the treatment.
"The Patterson case has done a lot to increase awareness of phage therapy and its effectiveness as an alternative therapy for multidrug-resistant pathogenic strains," Liu said. "The success of phage therapy in that case and other cases has brought wider attention to its use and efficacy."
Liu added that the Center for Phage Technology is focusing on developing the technology, standardizing optimal delivery procedures and securing necessary approvals from regulatory agencies to make phage treatment available to patients in the U.S.
"Much of what we did in the Patterson case was unconventional due to the context of phage therapy at that time," Liu said. "But there have been many advances in genomic sequencing and other technologies since then. Today, it would be a much quicker and more efficient process to develop and implement phage therapy if there was another case similar to Patterson's."
More information: Mei Liu et al, Comparative genomics of Acinetobacter baumannii and therapeutic bacteriophages from a patient undergoing phage therapy, Nature Communications (2022). DOI: 10.1038/s41467-022-31455-5