This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Prelude' to neuromuscular disease spinal muscular atrophy may offer chances for better treatment
Spinal muscular atrophy (SMA) is a severe neurological disease for which there is presently no cure, although current therapies can alleviate symptoms. In the search for better treatment options, scientists at DZNE and the Dresden University of Technology are now drawing attention to previously unnoticed abnormalities in embryonic development. They base their argument on studies of so-called organoids: laboratory-grown tissue cultures that can reconstruct disease processes.
Their findings are published in the journal Cell Reports Medicine.
In SMA, neurons in the spinal cord degenerate, leading to paralysis and muscle wasting. The disease usually manifests in childhood and affects an estimated 1,500 individuals in Germany. Defects in a specific gene are considered to trigger SMA. These mutations result in a deficiency of the so-called SMN protein (survival of motor neuron protein), which is critical for neurons involved in motor control.
For a number of years, medical treatments have been available to address the protein deficiency by means of gene therapy. Intervention can begin within a few days after birth. However, while this approach can alleviate disease symptoms, experience to date indicates that it provides no cure.
A so far unknown prelude
Now, scientists in Dresden, Germany, are suggesting broadening the perspective in the search for better therapies.
"The current perception of SMA focuses on the disease after birth, when the basic framework of the nervous system is mostly formed. This view ignores that phenomena relevant to the disease could occur much before, when the nervous system is still developing," says Dr. Natalia Rodríguez-Muela, a research group leader at DZNE's Dresden site and at the Center for Regenerative Therapies Dresden (CRTD) of Dresden University of Technology.
"In fact, our studies suggest that SMA is associated with anomalies in embryonic development not known until now. We therefore believe that there is a hitherto unrecognized prelude to this disease, and that interventions are needed that go beyond existing therapies."
Tiny pieces of tissue
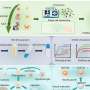
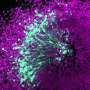
For their studies, Rodríguez-Muela and colleagues created "organoids" that recapitulate key features of both spinal cord and muscle tissue. These complex, albeit tiny samples of artificially generated tissue, each of them about the size of a grain of rice, were grown from human induced pluripotent stem cells. These, in turn, had been obtained by reprogramming skin cells of individuals affected by SMA.
"It is the first time that organoids of this complexity have been generated for studying SMA," Rodríguez-Muela says. "Although these are model systems that have certain limitations, they come quite close to the real situation, because they comprise a diversity of cell types and tissue structures that occur in the human body."
As the organoids matured over time, the scientists were able to study various developmental stages. "The earliest phase we can emulate with our organoid model corresponds to that of a human embryo a few weeks old. However, we only replicate the spinal cord and muscle tissue. Starting from the early developmental phase, we can go up to the situation after birth, in particular as it is observed in patients with SMA," Rodríguez-Muela explains.
Cellular aberrations
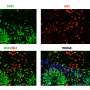
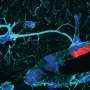
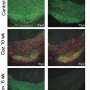
When the scientists compared organoids with SMA pathology with healthy specimens, they found significant differences: Specifically, stem cells in SMA organoids tended to develop prematurely into spinal cord neurons. In addition, there was a distortion in the cell population, i.e., fewer neurons than normal, which also were highly vulnerable, and more muscle cells derived from stem cells. Rodríguez-Muela and coworkers observed similar effects in mouse embryos with SMA-like pathology, supporting the findings in organoids.
These tissue cultures also yielded another important result.
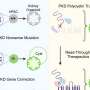
"When we corrected the genetic defect associated with SMA, we still observed developmental abnormalities, although to a lesser extent," says Rodríguez-Muela. "This suggests that restoring the gene, as current therapies kind of do, is most likely not enough to completely amend SMA pathology. This is in line with clinical experience to date. Thus, I believe, we need to address the developmental abnormalities if we want to improve treatment for SMA."
Spotlight on regulation
Rodríguez-Muela suspects that the cause for the observed developmental defects could lie in impaired gene regulation.
"It may not only be a question of whether the gene producing the SMN protein is defective or not. Perhaps it is also relevant [whether] the deficiency of this protein impacts other genes critical for the embryo's early development. There could be a regulatory effect. The fact is that we still don't know, but it is a plausible possibility," she says.
"I believe that this idea should be explored further. In the long term, this may lead to improved therapies that combine existing approaches with drugs targeting gene regulation. That is, they would have to act on what is called 'epigenetics.' In order to minimize the developmental abnormalities, such treatment would most likely need to be applied in early pregnancy. If prenatal testing indicates SMA, this could be a therapeutic option."
More information: Isogenic patient-derived organoids reveal early neurodevelopmental defects in spinal muscular atrophy initiation, Cell Reports Medicine (2024). DOI: 10.1016/j.xcrm.2024.101659