Research team reports on new treatment option for brain tumors that relapse or fail to respond to standard of care
Taken twice daily, oral olutasidenib helped to stabilize relapsed or refractory gliomas in heavily pretreated patients with less toxicity than standard of care treatment, according to a study led by Sylvester Comprehensive Cancer Center at the University of Miami School of Medicine and published in Neuro-Oncology.
Gliomas are the most common type of malignant brain cancer in adults and can affect young adults. Despite advances in treatment, which today include surgery, radiation, and chemotherapy, these tumors inevitably recur, progress and patients eventually succumb to the cancer. In addition to the dim prognosis, treatments are toxic to the brain and often leave patients with cognitive problems.
"There is a huge unmet need for effective, less-toxic glioma treatments," said the study's lead author Macarena de la Fuente, M.D., chief of the Division of Neuro-oncology at Sylvester. "Sylvester is a referral center for the treatment of glioma patients, and we have been working over the last eight years to identify a more efficacious and less toxic treatment for the care of these patients."
Dr. de la Fuente and colleagues from around the world studied 26 adult glioma patients harboring the isocitrate dehydrogenase protein 1 (IDH1) R132X mutation, which is among the more common mutations associated with the disease. Olutasidenib is a highly potent brain-penetrating drug that selectively inhibits the IDH1 mutation.
All the patients had undergone standard of care treatments but still had disease recurrence, so patients in the study received two daily doses of the oral drug and researchers followed their progress for an average of more than 15 months.
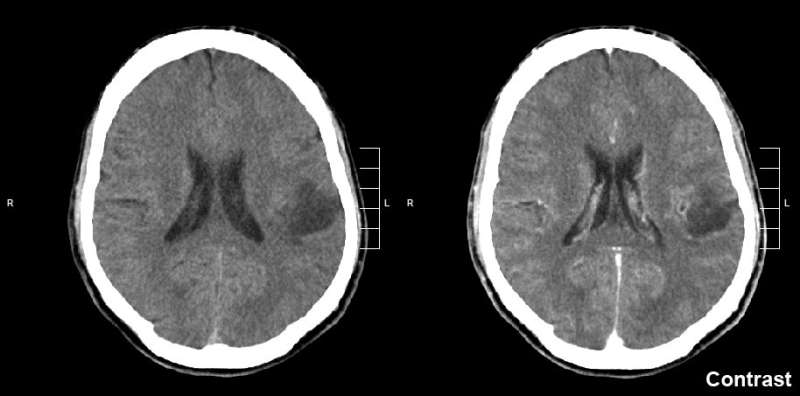
The investigators found that the drug helped control and stabilize the disease in nearly half of the patients. Eight of the patients, or 32%, had stable disease for at least four months. Additionally, two patients had a more than 80% reduction in tumor volume. The drug was well tolerated, with no dose-limiting toxicities reported.
"This is the first time that olutasidenib has been shown to be safe in a heavily pretreated glioma population and has demonstrated some preliminary efficacy," Dr. de la Fuente said. "We are moving forward by collaborating with our basic science researchers to find combinations that enhance efficacy while maintaining a benign toxicity profile."
More information: Macarena I de la Fuente et al, Olutasidenib (FT-2102) in patients with relapsed or refractory IDH1-mutant glioma: A multicenter, open-label, phase Ib/II trial, Neuro-Oncology (2022). DOI: 10.1093/neuonc/noac139