Researchers identify a key enzyme that controls white-to-brown fat conversion
Once considered an inert tissue, fat—or adipose tissue—is now known to play an active role in the body's critical functions by secreting hormones that regulate hunger and body temperature. Body fat comes in several varieties; for example, white adipose tissue stores excess energy, and brown adipose tissue primarily burns energy. In the last decade, researchers have isolated a new kind of fat tissue called beige adipose tissue. Beige fat cells begin life as white fat cells but take on the features of energy-burning brown fat cells under certain circumstances.
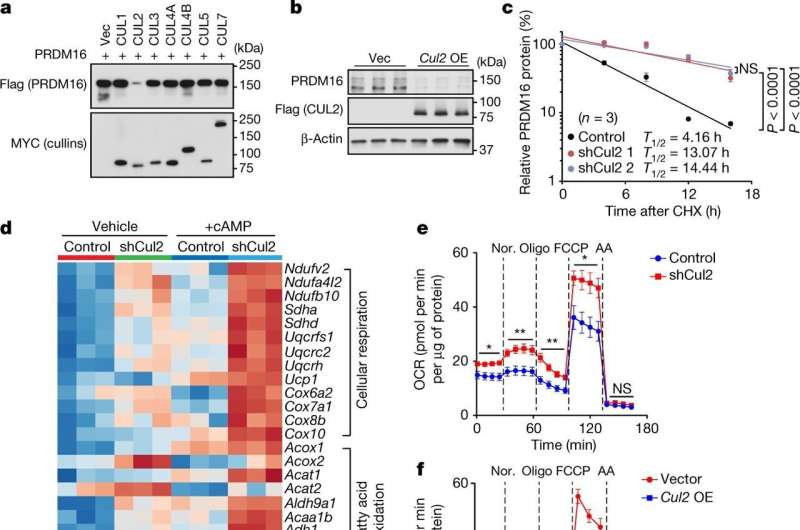
A growing body of evidence suggests that brown and beige adipose tissues are protective against metabolic disease, such as insulin resistance and diabetes. Increasing production, or biogenesis, of the energy-burning beige fat has been linked to substantial improvement in metabolic health. Now, researchers at Beth Israel Deaconess Medical Center (BIDMC) have developed a transformative approach to make more of this kind of cell. They identified a key enzyme, Cul2-APPBP2, which catalyzes the degradation of PRDM16 protein, a potent activator of beige fat biogenesis. The work is available online in the journal Nature.
"The most well-known approach to induce beige fat biogenesis is chronic cold acclimation; however, chronic cold acclimation may be unsafe due to elevated blood pressure, besides the fact that cold exposure is uncomfortable to most people," said corresponding author Shingo Kajimura, Ph.D., a Howard Hughes Medical Institute investigator in the Division of Endocrinology, Diabetes & Metabolism at BIDMC. "This work provides a completely new solution by suggesting a model of how beige adipocyte biogenesis is controlled."
Based on previous work suggesting that the protein PRDM16 played a key role in beige fat biogenesis, the researchers conducted a series of experiments to determine how the protein turnover was controlled in white fat cells in mice. A discovery ten years in the making, Kajimura and colleagues identified the key enzyme—an E3 ligase complex named CUL2-APPBP2—that breaks down the protein in fat cells.
In the absence of the E3 ligase complex, the PRDM16 protein activated the heat-producing genes that are characteristic of beige fat—counteracting diet-induced obesity, glucose intolerance, insulin intolerance and abnormal blood levels of fat in mice. The scientists also observed that the protein repressed damaging pro-inflammatory genes in the fat cells, suggesting another route by which brown and beige fat contribute to metabolic health.
"We hope that an inhibitor will be an effective approach to stimulate beige fat biogenesis and improve metabolic health safely," said first author Qiang Wang, an instructor in the Kajimura lab. "We are now searching for specific inhibitors to block the E3 ligase complex."
More information: Shingo Kajimura, Post-translational control of beige fat biogenesis by PRDM16 stabilization, Nature (2022). DOI: 10.1038/s41586-022-05067-4. www.nature.com/articles/s41586-022-05067-4