Zinc enhances albumin's protective role against Parkinson's disease
Revealing zinc's interaction with a critical transport protein underscores the need to study biological pathways under physiologically relevant conditions.
Heavy metals in the body have long been thought to induce the aggregation of disease-linked proteins, but a KAUST study shows this is not always the case.
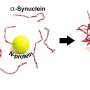
It turns out that zinc ions tune the ability of human serum albumin (HSA), an abundant transport protein in the body, to better prevent α-synuclein from aggregating, a process directly linked to Parkinson's disease.
The finding should "open new avenues for preventive treatments," says Samah Al-Harthi, a Ph.D. student who won a best poster award at the International Symposium on Frontiers in Molecular Science for the work.
The unexpected role for zinc discovered by Al-Harthi only became evident after she and her mentor, Łukasz Jaremko, studied the metal's effects on HSA, the main carrier of zinc in blood plasma and spinal fluid, under physiologically relevant conditions.
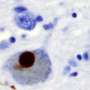
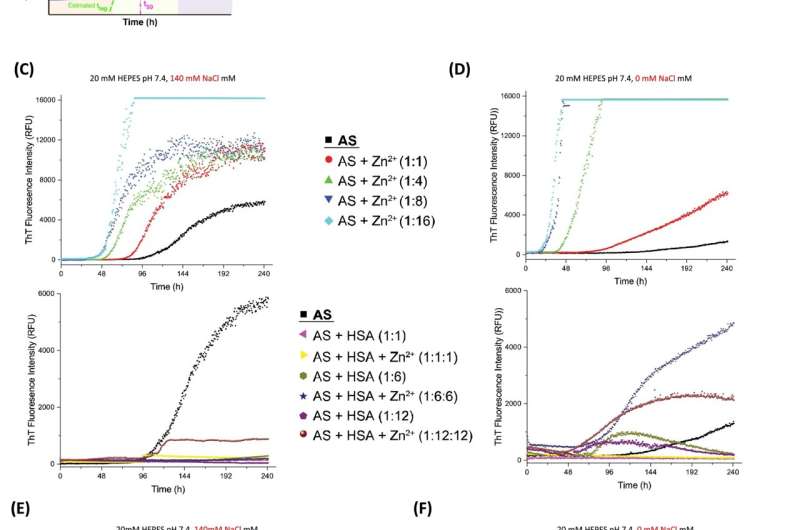
In unrealistically massive quantities, zinc tends to accelerate the aggregation of α-synuclein, a neuronal protein implicated in Parkinson's disease. This is what other scientists had shown in the past. But, under the types of biologically plausible experimental conditions considered by Al-Harthi, the metal actually interacts with HSA to cause the opposite effect.
Using a state-of-the-art imaging technique known as proton-less nuclear magnetic resonance spectroscopy, Al-Harthi and colleagues showed that zinc binding alters the chaperone function of HSA, a multifunctional molecule that plays a role in blocking α-synuclein accretion. In particular, zinc ions biased HSA toward stronger interactions with the aggregation-prone fragments of α-synuclein, a change that blunts fibril formation and slows down the toxic process of protein deposition that can lead to neurodegeneration.
The results dovetail with another recent report from Al-Harthi and Jaremko on the complex interplay between fatty acids, zinc ions and HSA. That report also required careful consideration of human physiology and experimental conditions to show how a particular fatty acid, one used regularly to treat diabetes, can help stabilize HSA function to address metabolic irregularities.
Together, the papers from Al-Harthi and Jaremko underscore the need to think about biological complexity when investigating intricate molecular pathways. "These medically relevant biomolecules cannot be studied in isolation if we want to discover medically relevant findings that can be of any future therapeutic use," Al-Harthi says.
"Biology is not so simple as the laboratory flask. This," she says, "is the driving force and motivation of my studies."
The recent papers are published in the International Journal of Biological Macromolecules and Frontiers in Chemistry.
More information: Samah Al-Harthi et al, Zinc ions prevent α-synuclein aggregation by enhancing chaperone function of human serum albumin, International Journal of Biological Macromolecules (2022). DOI: 10.1016/j.ijbiomac.2022.10.066
Samah Al-Harthi et al, Lipoic Acid Restores Binding of Zinc Ions to Human Serum Albumin, Frontiers in Chemistry (2022). DOI: 10.3389/fchem.2022.942585
Samah Al-Harthi et al, Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin, Journal of Inorganic Biochemistry (2019). DOI: 10.1016/j.jinorgbio.2019.110716