Shining a light on protein aggregation in Parkinson's disease
A novel system to control protein aggregation in a model of Parkinson's disease may answer longstanding questions about how the disease begins and spreads, according to a new study published March 9 in the open-access journal PLOS Biology by Abid Oueslati of Laval University, Quebec, Canada, and colleagues. Initial results suggest that aggregation of the protein alpha-synuclein plays a critical role in disrupting neuronal homeostasis and triggering neurodegeneration.
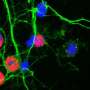
Parkinson's disease is a neurodegenerative disorder, marked clinically by tremor, stiffness, and slowed movements, as well as a host of non-motor symptoms. Within affected neurons, molecules of a protein called alpha-synuclein can be seen to clump together, forming characteristic aggregates called Lewy bodies. But it has been hard to answer whether alpha-synuclein aggregation contributes to disease development or progression, and when it may act in the toxic disease cascade, or whether instead the aggregates are innocent bystanders to some other malevolent process, or are even protective. These elements have been difficult to determine, in part because aggregation in cellular and animal models has not been controllable in either time or space.
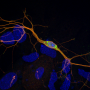
To address that problem, the authors turned to optobiology, a technique in which a protein of interest is fused to another protein that changes its conformation in response to light, allowing the behavior of the target protein to be manipulated selectively and reversibly. Here, the authors fused alpha-synuclein to a protein known as cryptochrome protein 2, from a mustard plant. They found that when light of the correct wavelength fell on the mustard protein, its conformational change triggered aggregation of its alpha-synuclein partner.
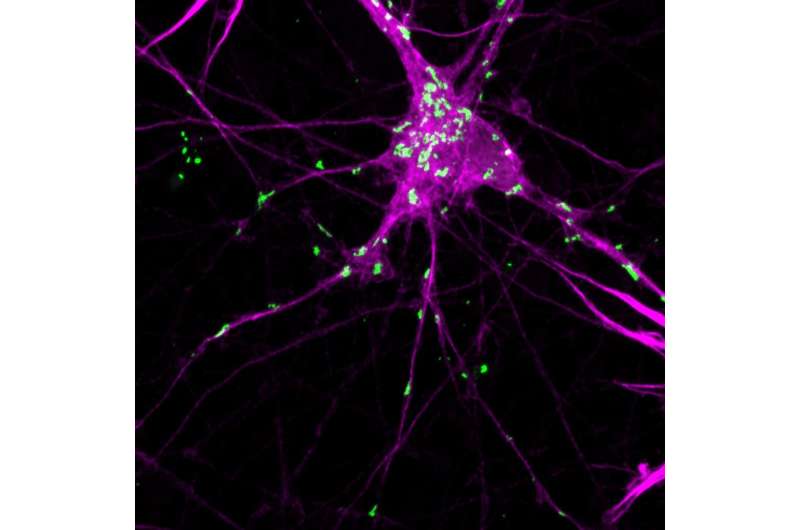
The aggregates that formed were reminiscent of Lewy bodies in multiple important ways, including that they included several other key proteins besides alpha-synuclein found in Lewy bodies in people with Parkinson's disease, and that the alpha-synuclein in the aggregates adopted the characteristic beta-sheet conformation seen in many diseases of misfolded proteins. The aggregates induced dislocation of multiple cellular organelles, as Lewy bodies have been recently reported to do as well. They also induced misfolding in alpha-synuclein molecules not attached to the cryptochrome protein, mimicking the prion-like spread of aggregation seen with alpha-synuclein in the diseased brain and animal models.
Finally, the authors delivered the genes for the alpha-synuclein-cryptochrome fusion protein to mice, directly into the substantia nigra, the structure in the brain that is most prominently affected by Parkinson's disease, and surgically placed an optic fiber to deliver light to the targeted cells. Light treatment led to formation of alpha-synuclein aggregates, neurodegeneration, disruption of calcium activity in downstream neuronal targets, and Parkinson-like motor deficits.
"Our results demonstrate the potential of this optobiological system to reliably and controllably induce formation of Lewy body-like aggregations in model systems, in order to better understand the dynamics and timing of Lewy body formation and spread, and their contribution to the pathogenesis of Parkinson's disease," Oueslati said.
Oueslati adds, "How do alpha-synuclein aggregates contribute to neuronal damage in Parkinson's disease? To help address this question, we developed a new optogenetic-based experimental model allowing for the induction and real-time monitoring of alpha-synuclein clustering in vivo."
More information: Morgan Bérard et al, A light-inducible protein clustering system for in vivo analysis of α-synuclein aggregation in Parkinson disease, PLOS Biology (2022). DOI: 10.1371/journal.pbio.3001578