New findings on how to avert excessive weight loss from COVID-19
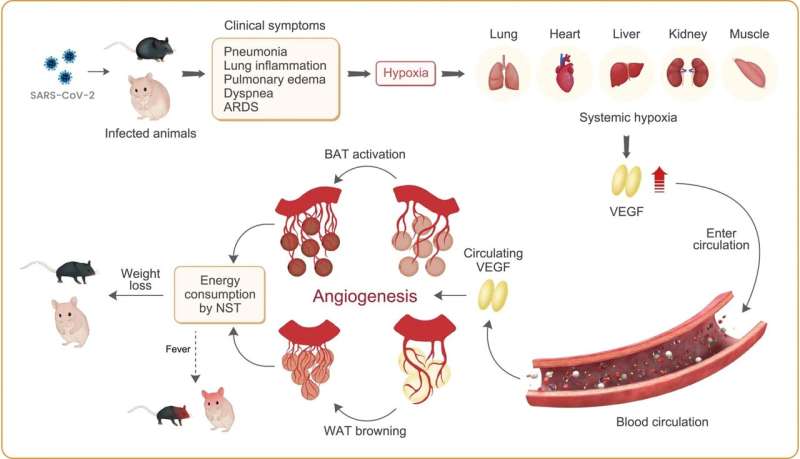
Losing too much weight when infected with COVID-19 has been linked to worse outcomes. Now, researchers at Karolinska Institutet in Sweden have discovered that SARS-CoV-2 infection fuels blood vessel formation in fat tissues, thus revving up the body's thermogenic metabolism. Blocking this process by using an existing drug curbed weight loss in mice and hamsters that were infected with the virus, according to the study published in the journal Nature Metabolism.
"Our study proposes a completely new concept for treating COVID-19 associated weight loss by targeting the blood vessels in the fat tissues," says Yihai Cao, professor at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, and the study's corresponding author.
The researchers examined how different types of fat, including brown fat and visceral and subcutaneous white fat, reacted when exposed to SARS-CoV-2 and how it impacted weight in mice and hamsters.
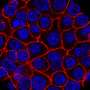
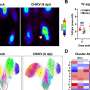
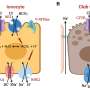
They found that the animals lost significant amounts of weight in four days and that this weight loss was preceded by the activation of brown fat and the browning of both types of white fat. These fat tissues also contained more microvessels and high levels of a signaling protein called vascular endothelial growth factor (VEGF), which promotes the growth of new blood vessels.
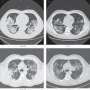
The researchers observed the same mechanisms in human tissue samples from four patients who died of COVID-19, suggesting the findings could be clinically relevant for humans.
When the researchers treated the animals with a substance that inhibits the formation of new blood vessels, a so-called antiangiogenic drug, the animals recovered most of their lost weight and their fat tissues exhibited fewer microvessels.
"Antiangiogenic drugs are currently used in the clinic to treat various types of cancers," Yihai Cao says. "It's possible these drugs could also be helpful in treating COVID-19-related problems such as excessive weight loss and metabolic changes, thus improving the quality of life and survival for these patients. Of course, we will need more research to validate if our preclinical findings also hold up in human trials."
More information: Yihai Cao, COVID-19 instigates adipose browning and atrophy through VEGF in small mammals, Nature Metabolism (2022). DOI: 10.1038/s42255-022-00697-4