Identifying pathways to slow cardiac aging

Cardiovascular disease is the leading cause of death worldwide, and is caused in part by age-related cardiac structural dysfunction. A team of bioengineers in Professor Adam Engler's lab at the University of California San Diego published a paper in Nature Aging on Dec. 22 that helps advance our understanding of how hearts age, and sheds light on a possible pathway to slow cardiac aging.
Natalie Kirkland, Ph.D., a postdoctoral scholar in Engler's Lab and first author of the paper, used fruit flies to show that Lamin C, a protein responsible for maintaining the structural integrity of heart cells' nuclei, declines as flies age. This study uncovered that Lamin decline is responsible for the age-induced structural remodeling in fruit fly hearts, and it could be a potential target to slow down, or even help reverse, cardiac aging in humans.
"Our work demonstrates that age-dependent nuclear remodeling plays a key role in cardiac function," Kirkland said "Nuclear morphology is likely a marker for cellular and tissue health and could be targeted for potential therapies."
Kirkland, researchers in the Engler Lab, and their collaborators at the Sanford Burnham Prebys Medical Discovery Institute and the National Institute on Aging at the National Institutes of Health, used fruit flies (Drosophila Melanogaster) for this study for four reasons:
- Fruit fly lifespan is between six and eight weeks, making them practical for age-related studies
- Fruit flies and humans share 82% of their cardiac proteomes.
- Studies in the 100s or 1000s are possible.
- Fruit flies have simple genetics that are easy to imitate.
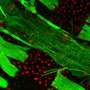
These properties make fruit flies a relatively quick and easy model to identify cardiac preserving pathways of interest for human research. Kirkland and fourth-year undergraduate bioengineering student Scott Skalak, a coauthor of the paper, used a microdissection technique on the flies' heart. The hearts were then preserved and examined with immunofluorescence and confocal microscopy.
"This is how I first observed nuclei were shrinking and becoming rounder in older flies," said Kirkland.
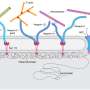
The team then quantified this change by segmenting and measuring nuclear stiffness with atomic force microscopy. This is when they discovered that cardiomyocyte nuclei stiffen during natural aging; after running a genetic analysis, the researchers found that the expression of nuclear lamins decreases as flies age.
The team was able to verify that these results also applied to mice and primates, thanks to collaborators at the National Institute on Aging. This indicates that a role for Lamins may apply to human heart aging as well, which could have tremendous therapeutic value, as targeting lamin-stimulating pathways could potentially help avoid this cardiac aging-related mechanical change.
"We found a role for cardiac transcription factors in regulating adult heart contractility and show that maintenance of Lamin C, and cardiac transcription factor expression, prevents age-dependent cardiac decline," the researchers write in the study. "Our findings are conserved in aged non-human primates and mice, demonstrating that age-dependent nuclear remodeling is a major mechanism contributing to cardiac dysfunction."
Future research will explore why lamins are lost with age, and how maintaining certain cardiac gene expression can improve heart function and lifespan.
For Skalak, the experience helped solidify his decision to apply to bioengineering Ph.D. programs after graduating in 2023. He learned how important planning and communication are to the research process, as well as a variety of molecular biology skills.
"The first few weeks and months of my involvement in the lab and this project involved me helping Dr. Kirkland where I could while simultaneously refining new skills I was taught," said Skalak. "These skills involved Drosophila husbandry and care, immunofluorescence, microdissection of Drosophila, and confocal microscopy. Over time I developed my new skills, especially Drosophila dissection, which allowed me to independently carry out new experiments. This led to the opportunity for me to perform experiments required for revision of the paper during early 2022."
More information: Natalie J. Kirkland et al, Age-dependent Lamin changes induce cardiac dysfunction via dysregulation of cardiac transcriptional programs, Nature Aging (2022). DOI: 10.1038/s43587-022-00323-8