January 26, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Experimental vaccine for deadly Marburg virus guards against infection with just a single dose
An experimental vaccine for Marburg virus—a deadly cousin of the infectious agent that causes Ebola—can protect large animals from severe infections for up to a year with a single shot, scientists have found in a new study.
Developed by the National Institute of Allergy and Infectious Diseases, along with collaborators at other institutions, the vaccine produces durable protection, a factor that underlines its promise for clinical translation and pandemic preparedness. So far its safety profile suggests that investigators may be on the brink of a vaccine that, in the not-too-distant future, may help control a Marburg virus outbreak.
The pathogen is extraordinarily virulent, one of the most lethal in the world—an infectious agent so dangerous that it's on lists of viruses with potential to be exploited in devastating acts of bioterrorism. It causes a severe infection that once was known as Marburg hemorrhagic fever, but now is widely referred to as Marburg virus disease. The pathogen belongs to the Filovirdae family, the same viral family as Ebolavirus.
Writing in the journal Science Translational Medicine, Dr. Ruth Hunegnaw, lead author of a new research paper on a series of studies testing an investigational vaccine in nonhuman primates, underscores the urgent need for measures that can prevent infection and control Marburg virus outbreaks.
"Marburg virus has been identified as a category A bioterrorism agent by the U.S. Centers for Disease Control and Prevention and a Category-A Priority Pathogen by the National Institute of Allergy and Infectious Diseases, needing urgent research and development of countermeasures because of the high public health risk it poses," Hunegnaw wrote in the journal.
The potential for deadly Marburg virus hotspots and full-blown outbreaks remain a genuine threat, especially on the continent of Africa where rare but lethal outbreaks episodically ignite human infections. As with Ebolavirus, it's posited that the Marburg infectious agent jumped the species barrier from bats to people and nonhuman primates. While bats live without harm from the pathogen, scientists at the World Health Organization estimate human mortality at 90%.
Between June 28 and September 16 of last year, Ghana's Ministry of Health was monitoring three confirmed cases of Marburg virus disease. All of the infected, two adults in their twenties and a baby, were from the same household. The 14-month old boy died within three days of hospital admission. His 26-year old father also died. The 24-year-old mother survived; however, health authorities identified a total of 198 contacts for the three family members. All contacts were monitored for 42 days.
Scientists at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland have taken significant steps toward making a vaccine against Marburg virus a reality. Working with collaborators at the National Emerging Infectious Diseases Laboratories of Boston University and the Department of Microbiology and Immunology at the University of Texas Medical Branch in Galveston, the researchers are already analyzing data from a human clinical trial. An investigational vaccine, like the one used in the animal tests, has been administered in a newly completed Phase 1 trial.
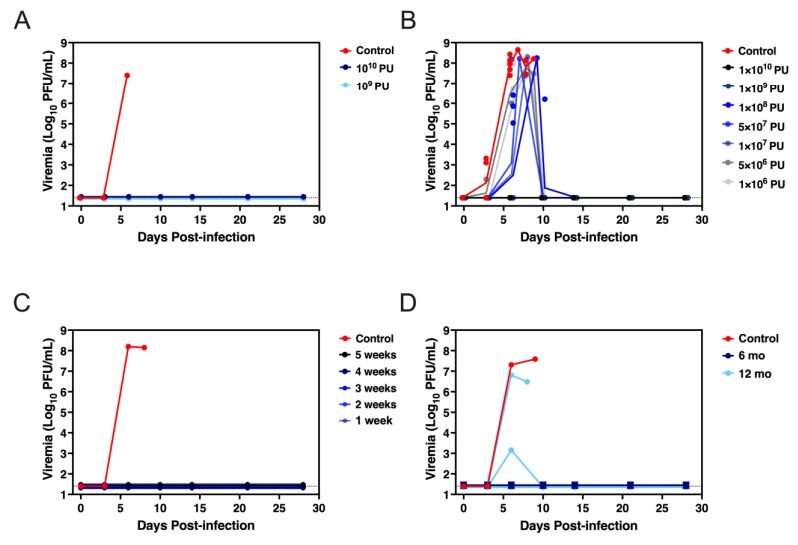
Hunegnaw, lead author of the research in nonhuman primates, reports that a single shot of the vaccine generated protective immunity within seven days of vaccination. Additionally—and perhaps more important—the investigational vaccine protected nonhuman primates when they were challenged with exposure to the lethal Marburg virus.
Hunegnaw and her colleagues note that the immunization is called ChAd3-MARV vaccine, an adenovirus-vectored shot that expresses the Marburg virus (MARV) glycoprotein. The harmless adenovirus in the vaccine is of chimpanzee origin, hence the initials "Ch" in the name of the vaccine.
"The recent cases of Marburg virus in West Africa underscore the substantial outbreak potential of this virus," Hunegnaw reported in Science Translational Medicine. "The potential for cross-border spread, as had occurred during the 2014–2016 Ebola virus outbreak, illustrates the critical need for Marburg virus vaccines."
Hunegnaw additionally reported that the animals remained protected from the pathogen when exposed to the virus a year after vaccination. The researchers also identified antigen-specific antibodies in the animals' blood, an important finding as scientists move toward regulatory approval by the U.S. Food and Drug Administration.
Marburg virus was first identified in 1967 when laboratory workers in Marburg and Frankfurt, Germany came down with a virulent hemorrhagic fever. The same infection was diagnosed in a Serbian laboratory worker in Belgrade. Scientists in all three locations were working with infected tissue samples from African green monkeys, Chlorocebus aethiops and were unaware of the exceptionally lethal nature of the virus. A total of 31 people were infected and seven died.
The experimental vaccine, meanwhile, which is being studied in Hunegnaw's laboratory and elsewhere in the United States, is seen as a major step toward fulfilling multiple goals—a vaccine for regions at risk of Marburg outbreaks and development of a vaccine in the event the virus is used in an act of bioterrorism.
"The demonstration of protection shortly after ChAd3-MARV vaccination and durability of the protection suggests that ChAd3-MARV is suitable for deployment both to protect healthcare workers during an outbreak and in a ring-vaccination scenario," Hunegnaw concluded.
More information: Ruth Hunegnaw et al, A single-shot ChAd3-MARV vaccine confers rapid and durable protection against Marburg virus in nonhuman primates, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abq6364
© 2023 Science X Network