This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Combining two bone healing remedies can inhibit bone growth and regeneration
Bone has the amazing capacity to regenerate. After a fracture, bone will heal without scarring in healthy people. In aging individuals, or for people with diseases like diabetes, approximately 10% of long bone fractures fail to heal well and require growth factors—or substances to help stimulate regeneration of specific tissues—to heal completely.
Two growth factors—bone morphogenetic protein 2 (BMP2) and platelet-derived growth factor (PDGF)—are currently approved by the Food and Drug Administration (FDA) to assist with bone healing. But combining them may make them less effective, Ivo Kalajzic, professor of reconstructive sciences and Sanja Novak, research instructor in the Center for Regenerative Medicine and Skeletal Development at UConn Health, report in the journal npj Regenerative Medicine. Their study evaluated the osteoanabolic—or bone forming—effects of PDGF BB and BMP2 when used together.
Bone morphogenic proteins are important regulators of bone regeneration, while platelet-derived growth factors help regulate cell growth and division. Alone, BMP2 helps increase bone mass and healing. But after evaluating mice treated with both PDGF and BMP2, the researchers found PDGF inhibits BMP2-induced bone growth.
"This is a clinically relevant study as BMP2 has been a widely used osteoanabolic in clinical practice and more studies are needed to understand how to improve its effects and lower the therapeutic dose to achieve the same or stronger bone healing effect," Kalajzic said.
To test the impact of both growth factors when combined, the researchers used multiple genetically modified mice and used microsurgical approaches to create bone fractures and "critical size" bone defects—or defects 20% of bone length. According to the researchers, the rodent model is ideal to understand the genetic regulation of bone healing.
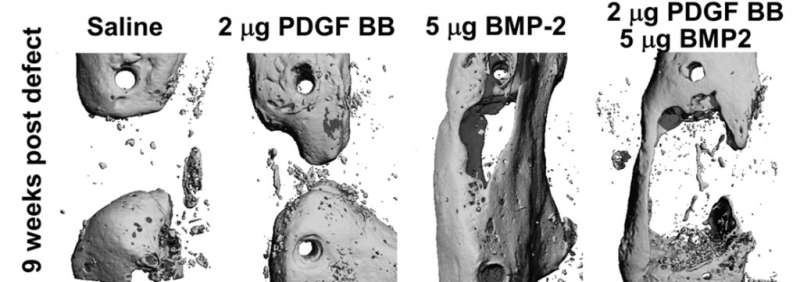
The researchers discovered the negative role of PDGF signaling in regulating BMP2-induced bone formation when placed within the bone defect. The combined treatment of critical sized defects early in the healing process decreased osteoprogenitor—or bone forming—cell numbers and limited the expansion of stem/progenitor cells, resulting in less bone formation nine weeks after the defect. However, inhibition of PDGF signaling early in the healing process did not lead to improved BMP2-induced osteogenesis, indicating PDGF is required for the healing process.
The study shows that two individual bone building agents, when combined, can actually decrease bone growth. The data shows how important timing and location are to bone growth factor effectiveness and proper healing.
The researchers said, "While BMP2 is usually clinically used, this study suggests that future studies are needed and other mechanisms need to be explored besides interactions between PDGF and BMP signaling to help induce bone regeneration and diminish significant side effects in patients."
More information: Sanja Novak et al, PDGF inhibits BMP2-induced bone healing, npj Regenerative Medicine (2023). DOI: 10.1038/s41536-023-00276-5