This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Evaluating equine immunoglobulin F(ab′) 2 for treatment of smallpox
Smallpox, a severe infectious disease caused by the smallpox virus, causes a death rate as high as 30% within 15–20 days after infection. Therefore, development of an anti-smallpox product as a strategic reserve is urgently needed.
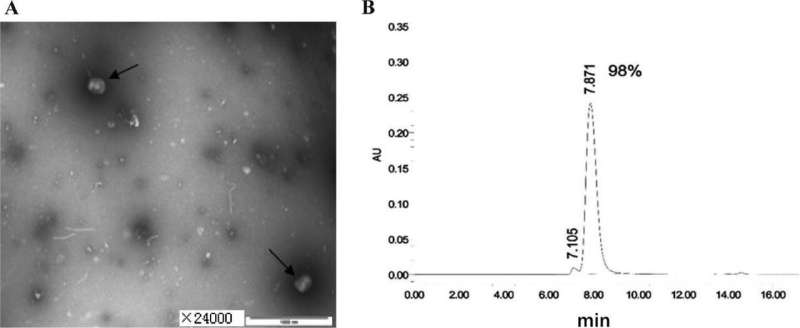
Pepsin-digested F(ab′)2 fragments of serum IgG from horses was prepared and tested by a team of researchers from China. Transmission electron microscopy indicated that the purified virus showed morphology consistent with VVTT. The titer was above 1.0 × 107 PFU/mL. The purity of the antigen exceeded 90%, according to HPLC.
After purification and cleavage, the yield of the purified product F(ab′)2 was approximately 1.3%, its purity exceeded 90%, and the neutralizing antibody titer exceeded 1:3200. F(ab′)2 fragments had good preventive and therapeutic effects in mice at antibody doses of 5.2 mg/mL and 2.6 mg/mL. The viral loads of the drug-treated mice were suppressed to varying degrees, and the higher dose groups (5.2 and 2.6 mg/mL) showed a 2–3 fold lower viral load than that in the control group.
A process for producing equine immunoglobulin F(ab′)2 against VVTT was established. The prepared horse anti-smallpox immunoglobulin product had good neutralizing antibody effects on VVTT. The highly purified preparation may serve as a potential candidate for smallpox treatment.
The study is published in the journal Zoonoses.
More information: Bochang Shi et al, Preparation of Equine Immunoglobulin F(ab′)2 against Smallpox and Evaluation of its Immunoprotective Effect, Zoonoses (2023). DOI: 10.15212/ZOONOSES-2022-0048



















