March 23, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Perivascular cells could induce microglial malfunction associated with Alzheimer's disease
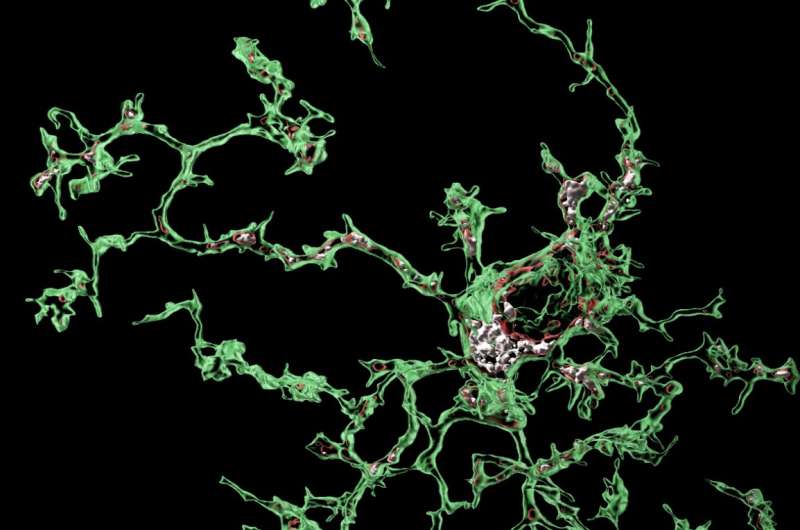
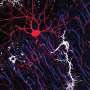
Microglia are primary immune cells that safeguard the mammalian brain, partly by devouring or 'phagocytosing' pathogens and toxic debris. Recent genetic studies have consistently highlighted the role of microglia in the development of Alzheimer's disease (AD) and other neurodegenerative diseases, indicating that they could aberrantly start phagocytosing synapses, the crucial connections between neurons.
Now, researchers at the UK Dementia Research Institute at University College London (UCL) carried out a study aimed at better understanding devouring processes through which microglia can increase the risk of developing AD.
"While the brain is developing, microglia phagocytose synapses that need to be removed for the brain to be properly wired," Dr. Soyon Hong, a group leader and corresponding author of the study, told Medical Xpress. "Yet when the brain suffers from diseases, or during aging, this crucial function of microglia could become reactivated, leading to the aberrant phagocytosis and loss of synapses. We were one of the first teams to show that microglia overeat synapses in a region-specific manner in mouse models that mimic some aspects of AD. This is relevant because synapse loss is one of the strongest correlates of cognitive impairment in human AD patients"
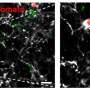
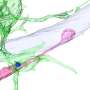
Although blocking microglial phagocytosis of synapses prevented synapse loss in AD mouse models, the signals that trigger this undesirable engulfment process remain a mystery. In this new study, published in Nature Neuroscience and led by postdoctoral scientist Dr. Sebastiaan De Schepper, the team unveiled an unprecedented communication between microglia and neighboring perivascular macrophages, a type of cell that lines small blood vessels, happening prior to the phagocytosis of synapses.
"We discovered the surprising role of perivascular macrophages in synapse pathology," Dr. De Schepper explained. "In the normal brain, perivascular macrophages patrol the blood for potential pathogens, but it now appears they also respond to early accumulation of amyloid beta around the vasculature."
Amyloid beta is a type of protein that is found in neuronal cells of the brain. When too much of this protein accumulates, it can form clumps called "plaques," which is a pathological hallmark of AD.
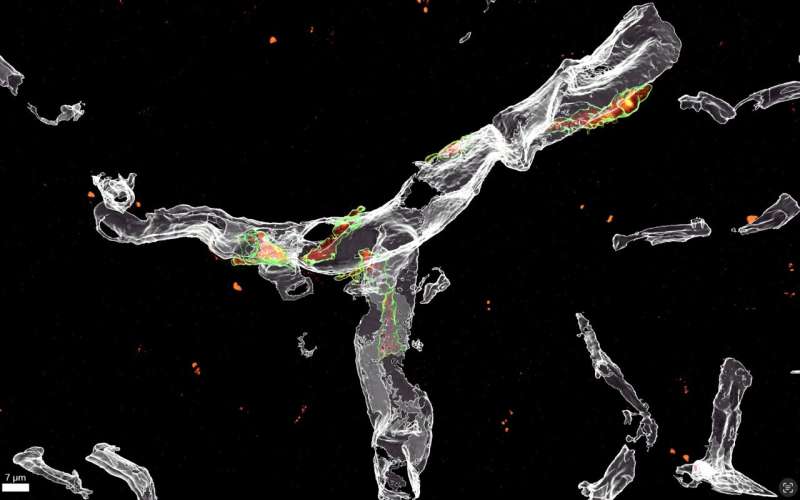
"It becomes increasingly clear however, that amyloid beta accumulates around blood vessels early in the disease, prompting local cells such as perivascular macrophages, to initiate an immune response," Dr. De Schepper added.
Using state-of-the-art microscopic techniques in mouse models of AD, Dr. De Schepper and Dr. Hong specifically found that this immune response entails the release of SPP1, or osteopontin, a glycoprotein that acts as an immune modulator, leading to activation of microglial engulfment of synapses.
"Notably, SPP1 has been found previously to be highly expressed in the cerebrospinal fluid of AD patients, but with unknown function until now," Dr. De Schepper further explained. "The release of SPP1 by perivascular macrophages might represent new therapeutic avenues to predict how the brain's immune system reacts towards early pathological alterations in AD."
An overreaching goal of the research conducted by the Hong lab at UCL is to pin-point the microglia subtypes contributing to the loss of synapses observed in patients with early-stage AD. Their new study was a further step in this direction, as it helped them to elucidate some of the processes involved in derailing microglia function.
"The identification and characterization of different microglia and perivascular subpopulations will have important therapeutic implications for the preservation of synapses in AD," Dr. Hong explained. "We hypothesize that one potential subtype could be characterized by high expression levels of the proinflammatory glycoprotein SPP1 (osteopontin). In the body, SPP1 marks many phagocytosing macrophage subtypes in multiple organs. In the brain, SPP1 also marks microglia and perivascular macrophages involved in phagocytosis, including that of synapses."
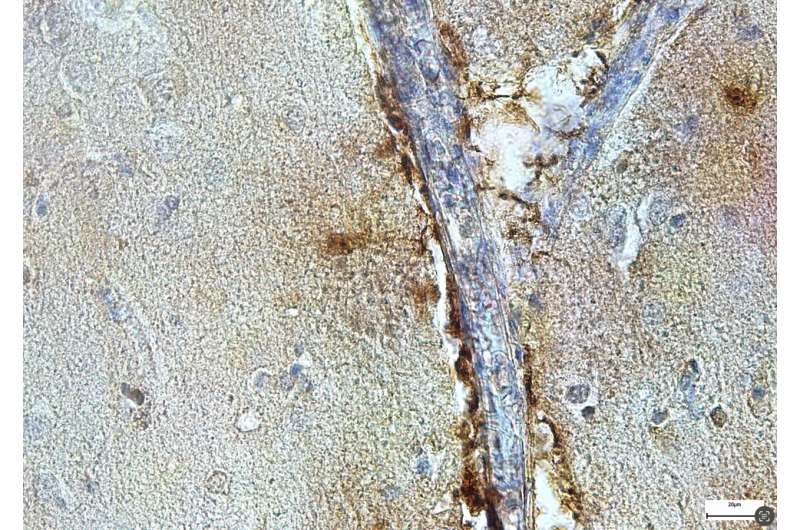
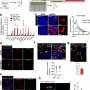
Dr. De Schepper and his colleagues then used single-cell RNA sequencing technology, a method that allows us to study the genes that are active in individual cells of the brain. They found that perivascular cells actively instruct microglia to phagocytose synapses via SPP1. In a next step, they used genetic techniques to remove the SPP1 gene from the mice. This allowed them to determine whether the absence of SPP1 could prevent the undesired process through which microglia engulf or eat vital synapses.
Indeed, microglia from mice that are deficient of SPP1 ('Spp1-Knock out mice') were prevented from engulfing synapses, indicating that the protein is required for microglial phagocytosis to take place. The recent results gathered by this team of researchers confirm previous findings hinting at the central role of the vascular space in AD.
"Our results support earlier studies that highlight vascular cells in the brain as a key site for the early formation of amyloid-beta protein deposits," Dr. Hong explained. "This could in turn trigger the increased production of SPP1, which could lead perivascular cells to signal neighboring microglia, inducing the overeating of synapses associated with AD."
Interestingly, in their study, Dr. De Schepper and team also detected SPP1 in perivascular cells within the hippocampus of deceased patients with AD, meaning that their results on mice could also apply to humans. Their findings could thus soon inform the development of new therapeutic interventions for AD, which are designed to modulate microglia activity via the perivascular space.
"We are now testing different ways to therapeutically target SPP1 in the early stages of AD, aiming to prevent synapse loss and inflammation in the brain," Dr. De Schepper added. "One way is via so-called 'antisense-oligonucleotides' (ASO). ASO are small pieces of genetic materials that are designed to bind to specific RNA molecules in the body. Together with the pharmaceutical company Ionis, we developed an ASO that specifically bind to SPP1 and interferes with its production, and we hope this will help to translate our findings into new therapeutic approaches for synapse preservation in AD."
More information: Sebastiaan De Schepper et al, Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer's disease, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01257-z
© 2023 Science X Network