This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Polyisoprenylated cysteinyl amide inhibitors deplete g-proteins in cancer cells
A new research paper was published in Oncotarget, titled, "Polyisoprenylated cysteinyl amide inhibitors deplete singly polyisoprenylated monomeric G-proteins in lung and breast cancer cell lines."
Finding effective therapies against cancers driven by mutant and/or overexpressed hyperactive G-proteins remains an area of active research. Polyisoprenylated cysteinyl amide inhibitors (PCAIs) are agents that mimic the essential posttranslational modifications of G-proteins. It is hypothesized that PCAIs work as anticancer agents by disrupting polyisoprenylation-dependent functional interactions of the G-Proteins.
In their new study, researchers Nada Tawfeeq, Jassy Mary S. Lazarte, Yonghao Jin, Matthew D. Gregory, and Nazarius S. Lamango from Florida A&M University College of Pharmacy Pharmaceutical Sciences and Imam Abdulrahman bin Faisal University tested this hypothesis by determining the effect of the PCAIs on the levels of RAS and related monomeric G-proteins.
"To investigate the hypothesized anticancer mechanisms of the PCAIs through disruption of G-protein function, we checked the effects of the PCAIs on the G-protein levels in lung cancer (A549 and NCI-H1299) and breast cancer (MDA-MB-231 and MDA-MB-468) cell lines," write the researchers.
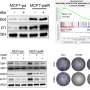
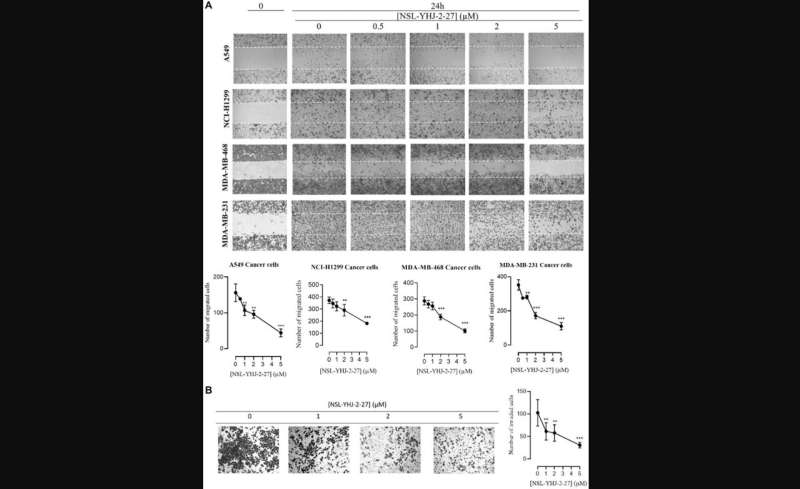
Following 48 hours of exposure, they found significant decreases in the levels of KRAS, RHOA, RAC1, and CDC42 ranging within 20–66% after NSL-YHJ-2-27 (5 μM) treatment in all four cell lines tested, A549, NCI-H1299, MDA-MB-231, and MDA-MB-468. However, no significant difference was observed on the G-protein, RAB5A.
Interestingly, 38 and 44% decreases in the levels of the farnesylated and acylated NRAS were observed in the two breast cancer cell lines, MDA-MB-231, and MDA-MB-468, respectively, while HRAS levels showed a 36% decrease only in MDA-MB-468 cells.
Moreover, after PCAIs treatment, migration, and invasion of A549 cells were inhibited by 72 and 70%, respectively while the levels of vinculin and fascin dropped by 33 and 43%, respectively. Their results show that PCAIs deplete the protein levels of some significant G-proteins which are known to be involved in the migration and invasion of cells (i.e., metastasis) such as RAC1, RHOA, and CDC42. These findings implicate the potential role of PCAIs as anticancer agents through their direct interaction with monomeric G-proteins.
"The initial findings presented here indicate how PCAIs can be used as potent agents in developing new anticancer therapeutics, therefore, more extensive studies need to be done to elucidate on its potency. Although we cannot conclusively explain the exact mechanism of action of PCAIs on how they affect the levels of some G-proteins yet, but we can say that these PCAIs have the ability to affect the progression of cancer," conclude the researchers.
More information: Nada Tawfeeq et al, Polyisoprenylated cysteinyl amide inhibitors deplete singly polyisoprenylated monomeric G-proteins in lung and breast cancer cell lines, Oncotarget (2023). DOI: 10.18632/oncotarget.28390