This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A safer method of generating pancreatic islet-like cells from human iPS cells
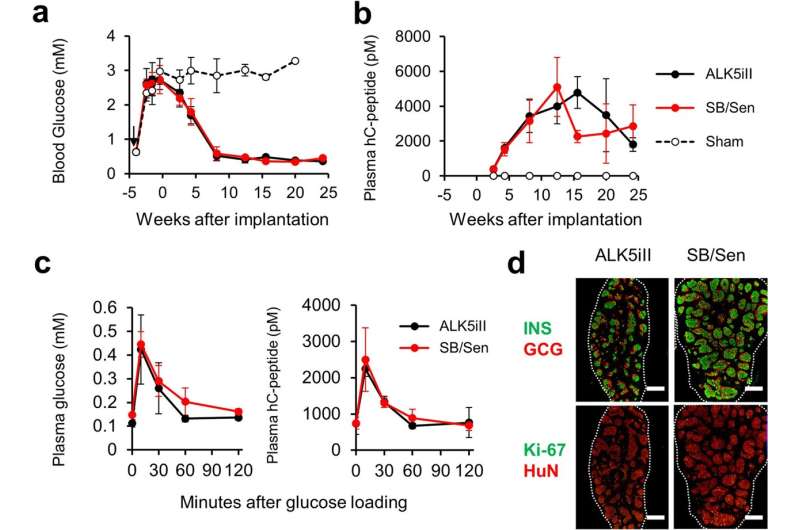
Junior Associate Professor Taro Toyoda and his research group, in collaboration with industrial companies including Orizuru Therapeutics, Inc., conducted a safety assessment of the differentiation inducers used in the preparation of human iPSC-derived pancreatic islet cells (iPIC) from human iPS cells and developed a safer differentiation protocol for generating iPICs based on those new findings. The research results were published in Stem Cell Research & Therapy on January 5, 2023.
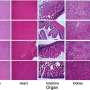
There are high expectations for the transplantation of pancreatic islet-like cells produced from human pluripotent stem cells like human iPS cells and ES cells, the goal of which is to revolutionize diabetes treatment. While it is possible to stringently evaluate and manage the risk posed by residual culture media components used during production by performing an impurity assessment on the final product, it is difficult to accurately assess the tumorigenic potential of the resulting islet-like cells in advance and conduct quality control.
In this study, the research group conducted a safety assessment of various differentiation inducers used to generate pancreatic islet-like cells from human iPS cells and found one reagent, ALK5 inhibitor II, to possess significant mutagenic potential.
During their attempts to replace the mutagenic ALK5 inhibitor II, they discovered that it has an inhibitory effect on cyclin-dependent kinase CDK8/19. The group further found that this CDK8/19 inhibition plays a crucial role in the differentiation process, leading them to establish a safer induction method to produce iPICs by switching to a combination of non-mutagenic CDK8/19 and ALK5 inhibitors.
These findings illuminate the underlying mechanisms involved in differentiating human pluripotent stem cells into islet-like cells and serve as an example of risk minimization against potentially harmful raw materials used in preparing cell therapy products.
More information: Kensuke Sakuma et al, CDK8/19 inhibition plays an important role in pancreatic β-cell induction from human iPSCs, Stem Cell Research & Therapy (2023). DOI: 10.1186/s13287-022-03220-4