This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Riluzole and sorafenib in patients with advanced solid tumors: A phase I trial
A new research paper titled "A phase I trial of riluzole and sorafenib in patients with advanced solid tumors: CTEP #8850" has been published in Oncotarget.
Overexpression of metabotropic glutamate receptor 1 (GRM1) has been implicated in the pathogenesis of multiple cancers. Riluzole, an inhibitor of glutamate release, showed synergistic antitumor activity in combination with the multi-kinase inhibitor sorafenib in preclinical models.
In a new phase I trial, researchers from Rutgers University, Dana-Farber Cancer Institute, and the Perlmutter Cancer Center of NYU Langone Health identified the toxicity profile, dose-limiting toxicities, maximum tolerated dose (MTD), and pharmacokinetic and pharmacodynamic properties of riluzole combined with sorafenib in patients with advanced cancers.
"Riluzole functions as an inhibitor of GRM1 signaling through antagonism of glutamate release, and sorafenib is a multi-kinase inhibitor targeting both the MAPK and PI3K/AKT pathways through the inhibition of RAF1, ARAF and, to a lesser extent BRAF, as well as a set of tyrosine kinases including VEGFR. Our phase I study determined the tolerable dose of this combination and investigated its biologic effects," the researchers write.
Patients with refractory solid tumors were enrolled utilizing a 3+3 dose-escalation design. Riluzole was given at 100 mg PO BID in combination with sorafenib, beginning at 200 mg PO daily and escalating in 200 mg increments per level in 28-day cycles. Restaging evaluations were performed every 2 cycles. In total, 35 patients were enrolled over 4 dose levels.
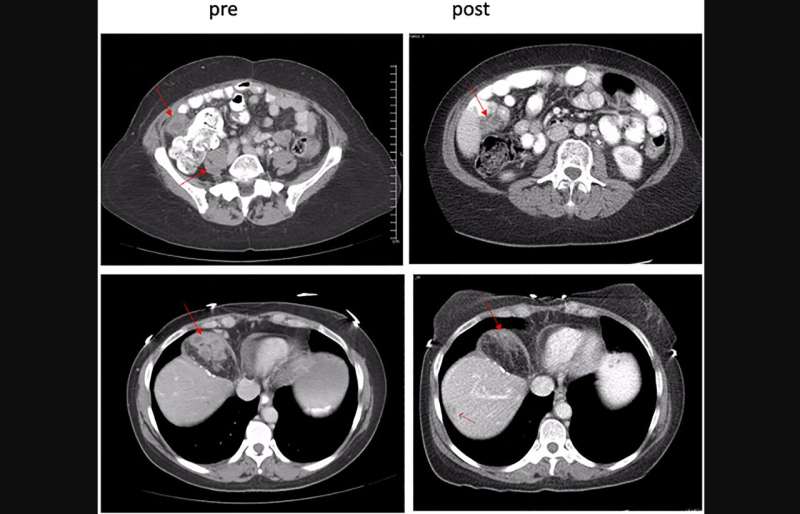
The MTD was declared at dose level 3 (riluzole: 100 mg PO BID; sorafenib: 400 mg AM/200 mg PM). Pharmacokinetic analyses did not reveal definitive evidence of drug-drug interactions. Consistent decreases in phospho-forms of ERK and AKT in tumor tissue analyses with accompanying decrease in GRM1 expression and increase in pro-apoptotic BIM suggest target engagement by the combination. Best responses included a partial response in 1 (2.9%) patient with pancreatic acinar cell carcinoma with a KANK4-RAF1 fusion, and stable disease in 11 (36%) patients.
The researchers conclude, "Combination therapy with riluzole and sorafenib was safe and tolerable in patients with advanced solid tumors. The partial response in a patient with a RAF1 fusion suggests that further exploration in a genomically selected cohort may be warranted."
More information: Kristen R. Spencer et al, A phase I trial of riluzole and sorafenib in patients with advanced solid tumors: CTEP #8850, Oncotarget (2023). DOI: 10.18632/oncotarget.28403