This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Mivavotinib for relapsed/refractory B-cell lymphoma
In a new research paper published in Oncotarget, researchers report an updated analysis from a phase I study of the spleen tyrosine kinase (SYK) and FMS-like tyrosine kinase 3 inhibitor mivavotinib. They present data for the overall cohort of lymphoma patients and the subgroup of patients with diffuse large B-cell lymphoma (DLBCL), including an expanded cohort not included in the initial report.
"Mivavotinib (TAK-659/CB-659) is an investigational, oral, reversible, potent dual inhibitor of spleen tyrosine kinase (SYK) and FMS-like tyrosine kinase 3 (FLT3). SYK is an essential component of the B-cell receptor signaling pathway; abnormal SYK signaling has been implicated in the pathogenesis of DLBCL and several other B-cell malignancies," say the researchers.
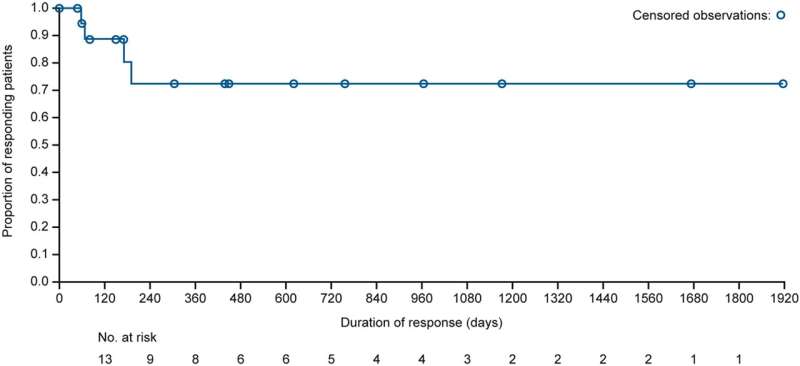
Patients with relapsed/refractory lymphoma for which no standard treatment was available received mivavotinib 60–120 mg once daily in 28-day cycles until disease progression/unacceptable toxicity. A total of 124 patients with lymphoma, including 89 with DLBCL, were enrolled. Overall response rates (ORR) in response-evaluable patients were 45% (43/95) and 38% (26/69), respectively. Median duration of response was 28.1 months overall and not reached in DLBCL responders.
In subgroups with DLBCL of germinal center B-cell (GCB) and non-GCB origin, ORR was 28% (11/40) and 58% (7/12), respectively. Median progression free survival was 2.0 and 1.6 months in the lymphoma and DLBCL cohorts, respectively. Grade ≥3 treatment-emergent adverse events occurred in 96% of all lymphoma patients, many of which were limited to asymptomatic laboratory abnormalities; the most common were increased amylase (29%), neutropenia (27%), and hypophosphatemia (26%).
"These findings support SYK as a potential therapeutic target for the treatment of patients with B-cell lymphomas, including DLBCL," note the researchers.
More information: Leo I. Gordon et al, Spleen tyrosine kinase/FMS-like tyrosine kinase-3 inhibition in relapsed/refractory B-cell lymphoma, including diffuse large B-cell lymphoma: updated data with mivavotinib (TAK-659/CB-659), Oncotarget (2023). DOI: 10.18632/oncotarget.28352