This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New tool charts differentiation landscape of acute myeloid leukemia
Researchers have developed a new method to distinguish between cancerous and healthy stem cells and progenitor cells from samples of patients with acute myeloid leukemia (AML), a disease driven by malignant blood stem cells that have historically been difficult to identify. The findings, published today in the journal Cell Stem Cell, pave the way for the development of new techniques to predict whether patients will respond to chemotherapy.
AML is a type of cancer characterized by the rapid growth and accumulation of abnormal white blood cells. It is thought to develop when blood progenitor cells, which normally turn into all other types of blood cells, fail to mature properly and become abnormal. In this process, blood stem cells carry a special importance because they give rise to progenitor cells and are thought to be the cell type in which leukemic mutations occur.
Leukemic stem cells are thought to survive chemotherapy and cause relapse. High relapse rates are a major clinical problem in AML and a frequent cause of patient death.
Understanding how blood stem cells give rise to blood progenitor cells in the context of AML is crucial for improving our understanding of the disease, developing better diagnostic and prognostic tools, and identifying new therapeutic targets and treatments. However, this has been historically difficult because of the high degree of variability between patients and the similarity between healthy and malignant stem cells.
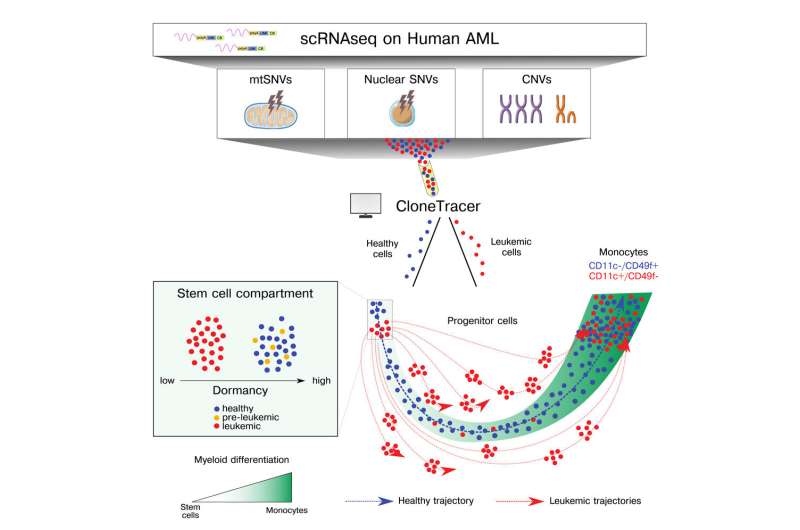
"Previous efforts to understand how leukemic stem cells and progenitor cells differentiate in AML have had mixed success because gene expression is very aberrant in the disease," explains Sergi Beneyto, first author of the paper and Ph.D. candidate at Dr. Lars Velten's research group at the Centre for Genomic Regulation (CRG). The authors set out to tackle this challenge by creating CloneTracer, a computational method.
The researchers used a technique known as single-cell RNA sequencing which measures the expression of genes in thousands of cells at the same time. They then applied CloneTracer to the data, which operates at clonal resolution, meaning it is able to trace the evolution of tumors by tracking how individual cells acquire mutations as they appear.
CloneTracer was used to analyze data from the bone marrow samples of 19 patients, revealing two distinct stem cell compartments; one predominantly healthy and dormant cluster of stem cells, and another compartment which was highly active and mostly consisted of leukemic stem cells.
CloneTracer also revealed that mutations, including seven of the ten most commonly-mutated AML driver genes, only prominently exert their effect in progenitor cells. The observed characteristics (phenotype) of these progenitor cells was correlated with the therapy response of patients.
The findings have implications for the prognosis and treatment of AML because progenitor cells that have differentiated to a more mature stage respond better to therapy.
"Once a leukemic cell starts differentiating into a progenitor, it goes crazy and becomes very different from what a healthy progenitor cell would look like. By looking at the progenitors, we can reasonably predict whether the first line chemotherapy will be successful. We are now working towards validating this in bigger cohorts and building clinical assays for these cell types," says Dr. Anne Kathrin Merbach, co-first author of the study and postdoc in the group of Prof. Carsten Müller-Tidow at the University Hospital of Heidelberg.
One of the limitations of CloneTracer is that single-cell RNA sequencing is costly, time-consuming and unfeasible to use in the clinic. Instead, the researchers plan on developing ways of characterizing the progenitor cells to predict response to chemotherapy by using FACS (fluorescence-activated cell sorting), a technique commonly used in leukemia research and which is available in most hematology medical departments around the world.
While predicting response to chemotherapy is important, the authors of the study acknowledge that unless targeted therapies also target the actual leukemic stem cell compartment the effect on relapse and long-term survival is limited. The clonal resolution offered by CloneTracer allowed the authors to characterize the gene expression signature of this critical cell population, which do not respond well to first-line chemotherapy. They caution that larger sample sizes will be needed to identify validate their findings before it can lead to improved therapies.
More information: Sergi Beneyto-Calabuig et al, Clonally resolved single-cell multi-omics identifies routes of cellular differentiation in acute myeloid leukemia, Cell Stem Cell (2023). DOI: 10.1016/j.stem.2023.04.001