This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves Xacduro for difficult-to-treat bacterial pneumonia
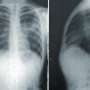
The U.S. Food and Drug Administration has approved Xacduro (sulbactam for injection; durlobactam for injection) as a new treatment for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia caused by Acinetobacter baumannii-calcoaceticus complex (A. baumannii).
Xacduro is administered via intravenous infusion and consists of sulbactam, a drug structurally related to penicillin, and durlobactam. It was approved under fast track, priority review.
The approval was based upon a multicenter active-controlled, open-label (investigator-unblinded, assessor-blinded), noninferiority clinical trial in 177 hospitalized adults with pneumonia caused by carbapenem-resistant A. baumannii. Nineteen percent of patients receiving Xacduro died versus 32 percent of those who received colistin. The most common adverse reaction associated with Xacduro was liver function test abnormalities.
"The FDA is dedicated to supporting the development of safe and effective treatment options for infections caused by difficult-to-treat bacteria like Acinetobacter baumannii-calcoaceticus complex," Peter Kim, M.D., director of the Division of Anti-Infectives in the FDA Center for Drug Evaluation and Research, said in a statement. "Today's approval helps address a high unmet medical need by providing an additional treatment option for some of the sickest patients in our nation's hospitals."
Approval of Xacduro was granted to Entasis Therapeutics.
More information: More Information
Copyright © 2023 HealthDay. All rights reserved.