This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers identify strong T-cell response in first-in-human nanoparticle HIV vaccine
Researchers from Fred Hutchinson Cancer Center in Seattle, Scripps Research in La Jolla, California, IAVI and other collaborating institutions have characterized robust T-cell responses in volunteers participating in the IAVI G001 Phase 1 clinical trial to test the safety and immune response of a self-assembling nanoparticle HIV vaccine.
Their work, published in Science Translational Medicine, signals a major step toward development of a vaccine approach to end the HIV/AIDS epidemic worldwide. The antigen used in this study was jointly developed by IAVI and Scripps Research and has been shown in previous analyses to stimulate VRC01-class B cells, an immune response considered promising enough for boosting in further studies.
"We were quite impressed that this vaccine candidate produced such a vigorous T-cell response in almost all trial participants who received the vaccine," explained Julie McElrath, MD, Ph.D., senior vice president and director of Fred Hutch's Vaccine and Infectious Disease Division and co-senior author of the study. "These results highlight the potential of this HIV-1 nanoparticle vaccine approach to induce the critical T-cell help needed for maturing antibodies toward the pathway of broadly neutralizing against HIV."
However, she added, this is the first step, and heterologous C will still be needed to eventually produce VRC01-class broadly neutralizing antibodies, which in previous studies have demonstrated the ability to neutralize approximately 90% of HIV strains.
"We showed previously that this vaccine induced the desired B-cell responses from HIV broadly neutralizing antibody precursors. Here we demonstrated strong CD4 T-cell responses, and we went beyond what is normally done by drilling down to identify the T cell epitopes and found several broadly immunogenic epitopes that might be useful for developing boosters and for other vaccines," William Schief, Ph.D., executive director of vaccine design for IAVI's Neutralizing Antibody Center at Scripps Research and professor, Department of Immunology and Microbiology, at Scripps Research, who is co-senior author of the study.
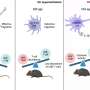
The trial is a phase 1, randomized, double-blind and placebo-controlled study to evaluate the safety and effectiveness of a nanoparticle HIV vaccine in healthy adult volunteers without HIV. It was comprised of two groups with 18 vaccine and six placebo recipients per group, with 48 total enrollees. Participants were given two doses of the vaccine or placebo eight weeks apart.
McElrath acknowledged the groundbreaking work of her lab team, the biostatistical team and Fred Hutch's Vaccine Trials Unit for their invaluable contributions to the study. The Vaccine Trials Unit conducts multiple vaccine trials and was one of only two sites for this study.
Findings from the study include:
- Vaccine-specific CD4 T cells were induced in almost all vaccine recipients.
- Lymph node GC T follicular helper cells increased after vaccination compared to placebo.
- Lumazine synthase protein, needed for self-assembly of the particle, also induced T-cell responses that can provide additional help to ultimately enhance efficacy in a sequential vaccine strategy.
- Vaccine-specific CD4 T cells were polyfunctional and had diverse phenotypes.
- LumSyn-specific CD8 T cells were highly polyfunctional and had a predominantly effector memory phenotype.
- CD4 T-cell responses were driven by immunodominant epitopes with diverse and promiscuous HLA restriction.
- CD8 T-cell responses to LumSyn were driven by HLA-A*02-restricted immunodominant epitopes B- and T-cell responses correlated within but not between LN and peripheral blood compartments.
More information: Kristen W. Cohen et al, A first-in-human germline-targeting HIV nanoparticle vaccine induced broad and publicly targeted helper T cell responses, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adf3309