This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study backs RSV vaccine safety during pregnancy

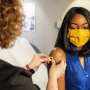
Vaccinating mothers against respiratory syncytial virus (RSV) during late pregnancy to protect their newborns is not associated with an increased risk of preterm birth or other poor outcomes, according to a study by Weill Cornell Medicine and NewYork-Presbyterian investigators. Infants are particularly vulnerable to the virus which can cause a serious lower respiratory illness.
The study published in JAMA Network Open on July 8 adds real-world evidence to the existing data from clinical trials about the safety of Pfizer's Abrysvo vaccine. The researchers found that there wasn't a significant statistical difference in preterm birth rates between vaccinated women (5.9%) and unvaccinated women (6.7%).
"The real-world evidence provides an additional layer of confidence about the safety of this vaccine during pregnancy," said the study's lead author Dr. Moeun Son, associate professor of obstetrics and gynecology at Weill Cornell Medicine. "Randomized clinical trials don't always emulate the populations we see in the clinical setting, but now we have data from multiple populations showing no increase in preterm birth risk."
The study's senior author is Dr. Heather Lipkind, professor of obstetrics and gynecology and director of Clinical Maternal-Fetal Medicine at Weill Cornell Medicine and a gynecologist-obstetrician at NewYork-Presbyterian/Weill Cornell Medical Center.
RSV protection from day one
Every year, RSV infections cause up to 80,000 hospitalizations and up to 300 deaths in children under five, according to the U.S. Centers for Disease Control and Prevention (CDC). For the first time last fall, mothers could reduce this risk— in August 2023, the U.S. Food and Drug Administration approved the Abrysvo vaccine for women between 32 and 36 weeks of pregnancy based on clinical trial results.
A month later, the CDC's Advisory Committee on Immunization Practices recommended the vaccine for eligible pregnant women during RSV season, September to the end of January. The single-dose shot stimulates production of protective antibodies that are transferred from mother to infant through the placenta.
To further study the vaccine's safety in actual practice, Dr. Son and her colleagues analyzed pregnancy outcomes in 1,026 vaccinated and 1,947 unvaccinated patients who received care at NewYork-Presbyterian/Weill Cornell Medical Center and NewYork-Presbyterian Lower Manhattan Hospital during the first year the vaccinations were available. All patient data was de-identified to protect privacy.
In addition to preterm birth rate, the team evaluated other birth risks in vaccinated and unvaccinated pregnant women. The two groups had similar rates of stillbirths, small-for-gestational-age birthweight, neonatal intensive care unit (NICU) admissions, respiratory distress with NICU admissions, jaundice, low blood sugar and sepsis among the newborns.
The data are particularly reassuring because they include some women with health conditions that could increase the risks of poor pregnancy outcomes who were excluded from the vaccine clinical trial.
"Patients and clinicians can feel confident that vaccination during pregnancy is a safe way to protect infants from harmful RSV infections," said Dr. Son, who is also a maternal-fetal medicine physician at NewYork-Presbyterian/Weill Cornell Medical Center.
Vaccination rates can be improved
To verify their results, Dr. Son and her colleagues conducted three separate analyses using different statistical methods. In one of the three analyses, they found a slightly increased risk of high blood pressure during pregnancy in vaccinated women. However, whether the difference in that analysis reflects a genuine concern or is the result of chance or differences between the two groups of women is still being determined. For example, vaccinated women were more likely to have insurance or to have undergone in vitro fertilization.
"These are things we will continue to explore in future studies," Dr. Son said.
The study also showed that the two New York City hospitals vaccinated 35% of the pregnant women under their care, which is almost double the national average of 18%. "We had onsite availability of the RSV vaccine in most of our prenatal outpatient clinics, which we think increased vaccination rates," Dr. Son said.
However, the researchers identified some disparities in vaccination rates. Black women, women with public insurance and those who had fewer prenatal visits were less likely to be vaccinated.
"As we prepare for the next RSV vaccination season in September, we are going to speak with different communities to help understand what might cause vaccine hesitancy among women or create barriers to access," Dr. Son said. "We want to ensure that all who would benefit will receive the vaccine."
More information: Moeun Son et al, JAMA Network Open (2024).