This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Barrett's esophagus modeled in a human organ chip
Acid reflux, the backwash of stomach acid into the swallowing tube, or esophagus, is something that many experience occasionally. When it happens repeatedly and becomes a chronic problem, it can result in "Barrett's esophagus" (BE), a condition in which cells in the epithelial lining of the esophagus appear to assume the identity of stomach or intestine cells.
This process is known as "metaplasia" and is associated with an increased incidence of esophageal cancer—one of the deadliest tumors estimated to be the sixth most common cause of cancer deaths worldwide.
To be able to predict which patients with Barrett's esophagus are at an increased risk for developing cancer, and to discover drugs that could prevent this transition, a much better understanding of its cellular and molecular triggers is needed. However, for studying BE, animal models are not useful, as they have a different esophageal anatomy, and existing in vitro models fail to replicate the changes in cell and tissue structure that are hallmarks of BE observed in human patients.
The esophagus is lined by a layer of epithelial cells that directly faces the esophageal lumen and is supported from below by a mixture of fibroblast cells and surrounding extracellular matrix known as "stroma." In addition to providing mechanical and nutritional support, it is known that the stroma sends signals to the epithelial cells to guide their differentiation and function during development of the esophagus in the fetus.
While the stroma continues to provide the esophageal epithelium with signals over a person's lifetime to enable tissue renewal, their contribution to the metaplasia that often precedes cancer formation remains unknown.
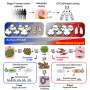
Now, a research team at the Wyss Institute for Biologically Inspired Engineering at Harvard University led by Wyss Founding Director Donald Ingber, M.D., Ph.D., in collaboration with researchers from McGill University in Montreal, Canada and University of California San Francisco (UCSF) developed a model that recapitulates the responses of a BE epithelium to stroma-derived fibroblasts in a patient-specific manner in vitro using Organ Chip technology.
Organ Chips are microfluidic cell culture devices in which key structures and functions of organs composed of different cell and tissue types can be mimicked outside of the body.
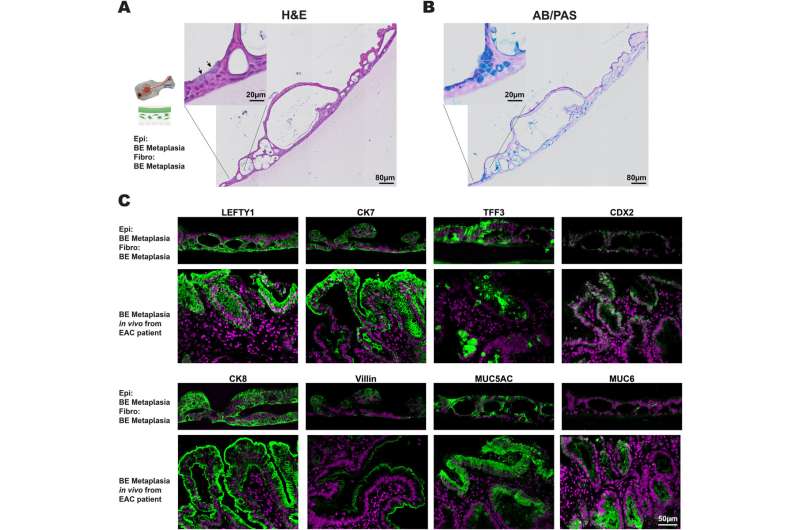
The team created chips lined by epithelial cells that were directly grown on a stroma-like layer with embedded fibroblast cells recreating an actual in vivo-like epithelial-stroma interface. Importantly, both the epithelial cells and fibroblasts were isolated from different regions within the same esophagus from a patient with esophageal cancer; some regions appeared healthy, while others showed typical signs of BE, or had progressed to cancer.
When they reconstituted the epithelial-stroma interface with epithelial cells and fibroblasts from a BE region, the cells reformed a tissue that strongly resembled BE tissue. But when they combined BE epithelial cells with fibroblasts from the cancerous regions, they observed that cells in the BE epithelial layer proliferated at a faster rate, and showed pre-cancerous changes under the microscope. The findings set the stage for in-depth analysis of the stroma-epithelial relationship in individual patients. They are published in Gastro Hep Advances.
Expanding the toolbox and personalizing it
In developing its BE Organ Chip model, Ingber's team built on previous Organ Chip models that they engineered to study the gastrointestinal system, including the colon; as well as organoid culture methods that they optimized for esophageal epithelial cells. By preserving patients' stem cell potential and differentiation into functional epithelial cells in organoids, the researchers created a long-lasting source for primary human epithelial esophageal cells.
"Many in vitro models of BE make use of patient cells that have been genetically manipulated to become immortalized, but they also lose some of their characteristics. Being able to apply primary human cells to Organ Chips allows us to stay much closer to the human situation," said first-author Elee Shimshoni, Ph.D., who worked on the project as a Postdoctoral Fellow on Ingber's team.
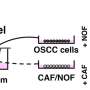
To mimic the human epithelium-fibroblast interactions and be able to observe them over an extended period of time, Shimshoni and her colleagues cultured the patient's fibroblasts within a collagen gel resembling the cells' natural extracellular matrix in the top channel of an organ chip that contains two parallel channels separated by a porous membrane.
To facilitate direct contact between the two cell populations, the esophageal epithelial cells, taken from their organoid cultures, were grown on top of the fibroblast-containing collagen gel in the top channel. A removable lid on this Organ Chip version allowed the epithelial cells to be exposed to air at a so-called air-liquid interface, which is important for their complete differentiation and formation of a tight epithelial layer, just as it happens in the esophagus in vivo. Through the lower channel, liquid medium was continuously flowed to provide nutrients to the cultured cells.
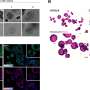
When the researchers combined normal fibroblasts with normal epithelial esophageal cells from a healthy-appearing region in what they called a "homotypic pairing," this resulted in a multilayered epithelium with normal esophageal cell morphology and composition, and normal molecular marker expression.
On the other hand, a homotypic pairing of BE-associated fibroblasts with BE epithelial cells produced a BE-like epithelium with thicker cuboidal epithelial cells and interspersed mucus-producing goblet cells usually found in the intestine. These findings showed that their culture system was able to recapitulate normal and BE esophageal tissue formation.
However, "only when we paired the BE epithelial cells with esophageal cancer-associated fibroblasts from a cancerous site in the same individual's esophagus in a 'heterotypic pairing,' were BE epithelial cells pushed into a hyper-proliferative state typical of a pre-cancerous process—normal fibroblasts and BE-specific fibroblasts could not produce this effect in heterotypic and homotypic pairings."
Potential for impacting patients' lives
These models enable investigators to not only study the molecular and cellular basis of epithelial-stromal interactions that play key roles in the progression from metaplasia to cancer formation, but also interactions guiding normal esophagus development in a highly-relevant tissue context. "To our knowledge, this is the first in vitro system in which it is possible to analyze the heterogenous responses of BE epithelium to stromal cells from different regions of the same organ from the same patient," said senior author Donald Ingber, M.D., Ph.D.
"This approach, if done for multiple patients, could allow us to identify early biomarkers of changes that are indicative of cancer progression, and perhaps even future therapeutic targets. It also has potential to be used to personalize the selection of therapeutics."
Ingber's team, spear-headed by Shimshoni, worked closely with the groups of Thea Tlsty, Ph.D. at UCSF, and clinical scientist Lorenzo Ferri, M.D., Ph.D. at the McGill University Health Centre in Canada.
Ferri is the Head of the Center's Division of Thoracic and Upper Gastrointestinal Surgery treating patients with esophageal and other cancers and malignancies and provided access to cell type-specific samples from a patient with esophageal cancer, while Tlsty has been extensively studying the contributions of stroma to wound healing and malignancies in multi-disciplinary approaches. The groups are part of a consortium that focuses on the key role that stroma plays in the progression of metaplastic tissues to cancers in various organs.
More information: E. Shimshoni et al, Epithelial-Stromal Interactions in Barrett's Esophagus Modeled in Human Organ Chips, Gastro Hep Advances (2023). DOI: 10.1016/j.gastha.2023.03.009