This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Cellular senescence involves gene repression through p53-p16/RB-E2F-DREAM complex
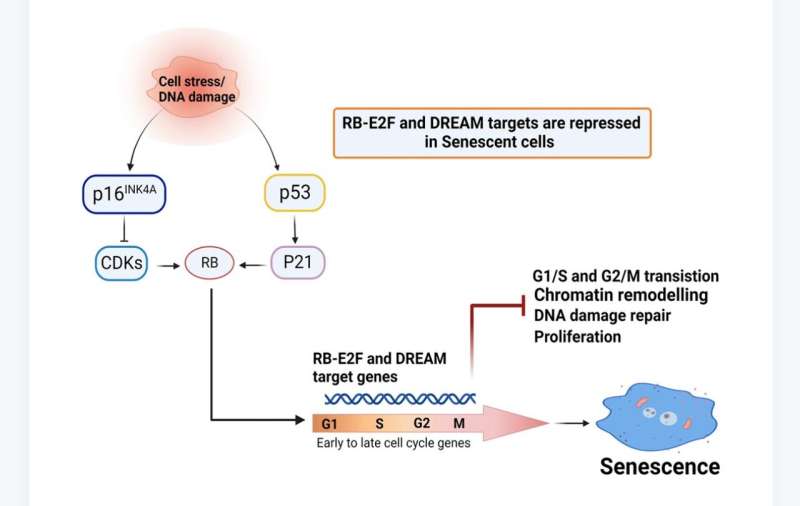
A new research paper titled "Key elements of cellular senescence involve transcriptional repression of mitotic and DNA repair genes through the p53-p16/RB-E2F-DREAM complex" has been published in Aging.
Cellular senescence is a dynamic stress response process that contributes to aging. From initiation to maintenance, senescent cells continuously undergo complex molecular changes and develop an altered transcriptome. Understanding how the molecular architecture of these cells evolve to sustain their non-proliferative state will open new therapeutic avenues to alleviate or delay the consequences of aging.
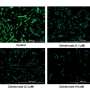
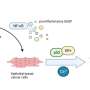
In this new study, seeking to understand these molecular changes, researchers Renuka Kandhaya-Pillai, Francesc Miro-Mur, Jaume Alijotas-Reig, Tamar Tchkonia, Simo Schwartz, James L. Kirkland, and Junko Oshima from the University of Washington, Vall d'Hebron Research Institute (VHIR), Vall d'Hebron Hospital, and the Mayo Clinic studied the transcriptomic profiles of endothelial replication-induced senescence and senescence induced by the inflammatory cytokine, TNF-α. The researchers had previously reported gene expressional pattern, pathways, and the mechanisms associated with upregulated genes during TNF-α induced senescence.
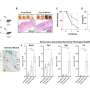
"Here, we extend our work and find downregulated gene signatures of both replicative and TNF-α senescence were highly overlapped, involving the decreased expression of several genes associated with cell cycle regulation, DNA replication, recombination, repair, chromatin structure, cellular assembly, and organization," the team explains.
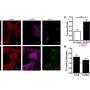
The team identified multiple targets of p53/p16-RB-E2F-DREAM that are essential for proliferation, mitotic progression, resolving DNA damage, maintaining chromatin integrity, and DNA synthesis that were repressed in senescent cells. They show that repression of multiple target genes in the p53/p16-RB-E2F-DREAM pathway collectively contributes to the stability of the senescent arrest. Their findings show that the regulatory connection between DREAM and cellular senescence may play a potential role in the aging process.
The researchers conclude, "This study suggests that the transcriptome signature of senescent cells goes beyond cell cycle arrest, with expression of multiple genes, from cell cycle to DNA repair to chromatin structure, being coordinately repressed to stably lock cells into this essentially non-proliferative state."
More information: Renuka Kandhaya-Pillai et al, Key elements of cellular senescence involve transcriptional repression of mitotic and DNA repair genes through the p53-p16/RB-E2F-DREAM complex, Aging (2023). DOI: 10.18632/aging.204743