This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves Litfulo for teens, adults with alopecia areata
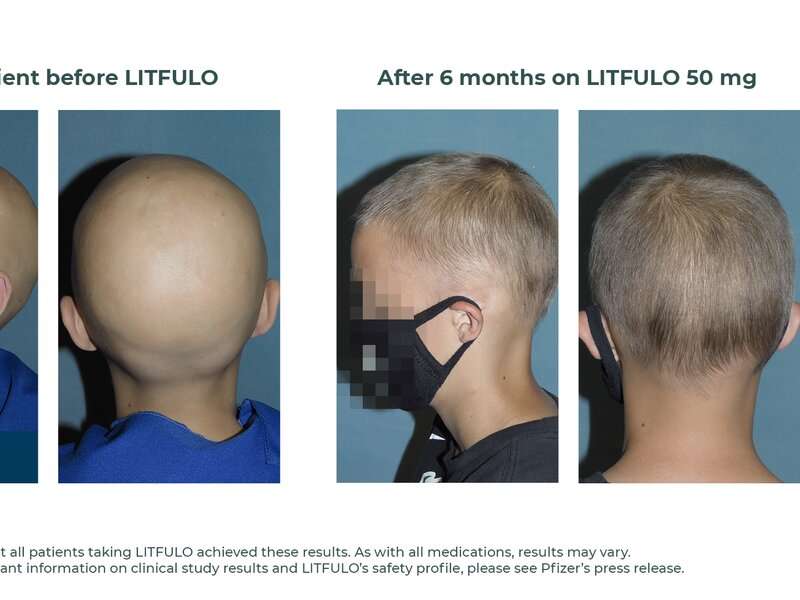
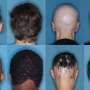
The U.S. Food and Drug Administration has approved Litfulo (ritlecitinib) for adults and adolescents aged 12 years and older with severe alopecia areata at a recommended dose of 50 mg. Approval of Litfulo was granted to Pfizer.
Litfulo is an inhibitor of Janus kinase 3 and the tyrosine kinase expressed in the hepatocellular carcinoma family of kinases, and may block signaling of cytokines and cytolytic activity of T cells.
The FDA approval was based on the results of clinical trials involving patients with alopecia areata, including the ALLEGRO Phase 2b/3 trial, which assessed the efficacy and safety of Litfulo in 718 patients from 118 sites in 18 countries. In this trial, significantly more patients treated with Litfulo 50 mg had 80 percent or more scalp hair coverage after six months compared with placebo (23 versus 1.6 percent). The efficacy and safety of Litfulo was consistent for both adolescents (aged 12 through 17) and adults (aged 18 and older).
"Litfulo is an important treatment advancement for alopecia areata, an autoimmune disease that previously had no FDA-approved options for adolescents and limited options available for adults," Angela Hwang, Chief Commercial Officer of Pfizer, said in a statement. "With today's approval, adolescents and adults who struggle with substantial hair loss have an opportunity to achieve significant scalp hair regrowth."
More information: More Information
Copyright © 2023 HealthDay. All rights reserved.