This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study identifies novel regulators of hippocampal overactivity
Reduced expression of specific calcium channels impaired electrical signaling between neurons and contributed to the onset of behaviors associated with neurodevelopmental disorders and seizure susceptibility in mouse models, according to a Northwestern Medicine study published in the Proceedings of the National Academy of Sciences.
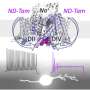
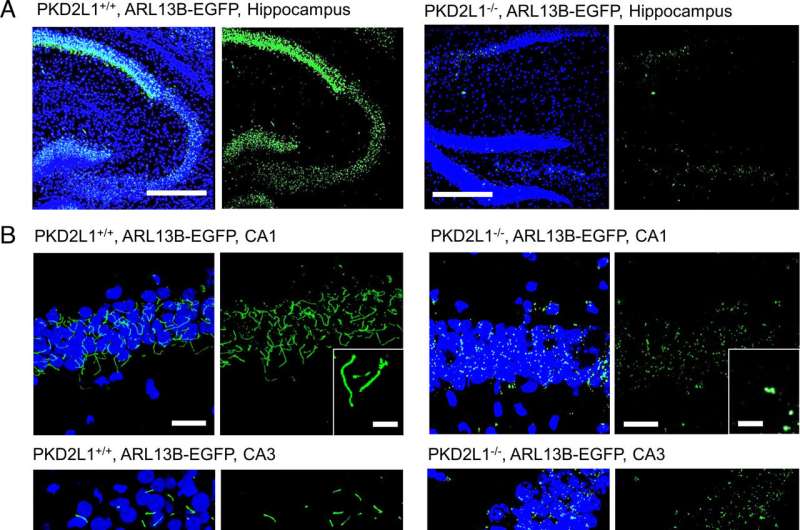
"Our results identify PKD2L1 channels as regulators of hippocampal excitability and the neuronal primary cilia as organelle mediators of brain electrical signaling," said Paul DeCaen, Ph.D., associate professor of Pharmacology and senior author of the study.
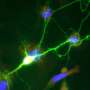
Polycystins are a group of proteins that include PKD2, PKD2L1, and PKD2L2, and localize to structures called primary cilia. These antenna-like organelles, which have previously been studied in additional work from the DeCaen laboratory, protrude from the surface of cells and help transmit electrical signals from one cell to another.
Most notably, PKD2 mutations are known to cause cystic kidney disease, which occurs when fluid-filled cysts grow in the kidneys and causes the organs to enlarge and lose function over time.
Interestingly, other previous work has shown that PKD2L1 is expressed in the brain, but the impact of PKD2L1 deficiency on neuronal function has remained understudied.
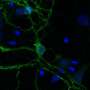
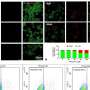
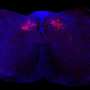
In the current study, DeCaen's team developed PKD2L1 knockout mouse models to track PKD2L1 expression and localization in the brain. Using electrophysiology and electroencephalography (EEG) techniques, the investigators discovered that PKD2L1 localizes and functions as a calcium ion channel in the primary cilia of neurons within the hippocampus, a region of the brain that plays a major role in learning and memory.
Furthermore, the loss of PKD2L1 expression inhibited the development of primary cilia, ultimately reducing electrical signaling and crosstalk between neurons. Reduced PKD2L1 expression in the mice was also associated with seizure susceptibility and the development of behaviors similar to neurodevelopmental disorders in humans.
Overall, the findings demonstrate PKD2L1 channels as regulators of hippocampal excitability and neuronal primary cilia as mediators of brain electrical signaling, both of which could serve as therapeutic targets for treating certain neurodevelopmental disorders, according to DeCaen.
The findings also open the door for further investigation into neuronal function and activation, DeCaen said.
"Everyone thinks of neurons as kind of wires where they have a synaptic cleft that will interact with another neuron, and when this fires some electricity, it then transmits it down the other neurons. What we're seeing now is not only that, but they also have this antenna-like structure which is beyond just a synapse. What are the inputs that trigger the electrical activity out here that transmits to the rest of the cell? Identifying what is the ligand or the activating stimulus for the channel in that little organelle is an open question," DeCaen said.
More information: Thuy N. Vien et al, Primary cilia TRP channel regulates hippocampal excitability, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2219686120