This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New model offers insights into how stress in neurons connects to cardiovascular disease
Oxidative stress—characterized by elevated levels of unstable molecules called reactive oxygen species—is associated with neurodegeneration and cardiovascular disease. However, until recently it has not been possible to demonstrate a causal relationship between oxidative stress and disease states.
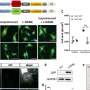
A new study used "chemogenetics" to activate a recombinant yeast protein expressed in mouse tissues to manipulate levels of oxidative stress in living mice. Researchers from Brigham and Women's Hospital, Harvard Medical School, and the Novartis Institutes for Biomedical Research applied chemogenetic approaches in a new transgenic mouse model to introduce oxidative stress into blood vessels and neurons.
The researchers initially set out to use this new transgenic mouse model to identify pathways through which oxidative stress might cause dysfunction of blood vessels and lead to diseases like hypertension and aortic aneurysms. But they were surprised to find that these mice rapidly developed profound ataxia, characterized by an inability to walk.
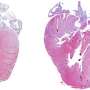
Probing further, they found that specific sets of sensory neurons in peripheral nerve cells had undergone degeneration from oxidative stress caused by the transgene. And when the researchers looked at the hearts of these animals, they found that heart muscle had developed cardiac hypertrophy.
This combination of sensory neuron degeneration and cardiac hypertrophy is associated with Friedreich's ataxia (FA), a progressive neurodegenerative disease that is the most common form of hereditary ataxia found in patients. Researchers also characterized specific inflammatory cell types involved in these responses, offering a more complete understanding of the mechanisms through which FA causes cardiac hypertrophy.
"Our team followed up on an unexpected phenotype that we uncovered in a new transgenic mouse line and found surprising new connections between peripheral nerves and the heart," said Thomas M. Michel, MD, Ph.D. of the Brigham Division of Cardiovascular Medicine. "Our findings may help us understand the cardiac remodeling seen in the hearts of patients with neurodegenerative diseases."
The study is published in the journal Nature Communications.
More information: Shambhu Yadav et al, Sensory ataxia and cardiac hypertrophy caused by neurovascular oxidative stress in chemogenetic transgenic mouse lines, Nature Communications (2023). DOI: 10.1038/s41467-023-38961-0