June 8, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Multi-omic signals associated with maternal epidemiological factors contributing to preterm birth

In low- and middle-income countries, and in general, the prospect of preterm birth is the leading cause of mortality in children under the age of five, although comprehensive studies are hindered to the phenomenon due to its complex etiology. Researchers have previously characterized the epidemiological associations between preterm birth and maternal characteristics.
In a new report now featured on the cover page of Science Advances, Camilo A. Espinosa and a team of scientists at Stanford Medicine, Stanford University, the Aga Khan University, Pakistan, and several other international research institutes used multi-omics profiling to investigate characteristic biological signatures.
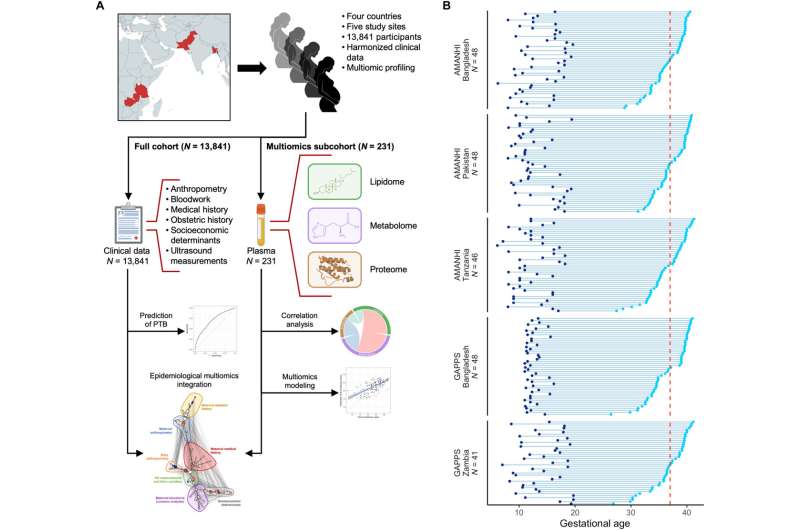
The researchers collected maternal covariates during pregnancy, from 13,481 pregnant women and analyzed plasma samples from patient subsets to generate proteomic, metabolomic and lipidomic (i.e., multi-omic) datasets. The scientists used machine learning models with robust performance to predict preterm birth, time-of-delivery, maternal age and the body-mass-index among a range of parameters.
The maternal age negatively correlated with collagen, gravidity with endothelial nitrous oxide, inflammatory chemokine, and the body-mass index was influenced by leptin and some structural proteins. The outcomes provided a full-view of epidemiological factors underlying preterm birth, while identifying biological signatures of related clinical covariates.
Maternal factors underlying preterm-birth
Birth prior to 37 weeks of gestation is deemed preterm and is a leading cause of mortality in children under the age of five around the globe. The occurrence has high prevalence in low and middle-income countries. Child-birth at preterm can increase the risk of life-threatening short-term complications, including long-term neurological, cardiovascular and metabolic morbidities.
This phenomenon affects the life of the mother, child and family, posing a high burden to global public health. The etiology of preterm birth is complex and carries a diverse range of effects to varying degrees.
While researchers have described the epidemiological associations between preterm birth and maternal clinical history, the outcomes are limited by a lack of biological data. Socioeconomic determinants of health are key players that affect the outcome of gestation, while environmental exposures can influence the health of a pregnancy; further complicating the risks of preterm birth.
During this work, the team used a multinational cohort of pregnant women data to understand epidemiologically derived factors associated with the phenomenon. The outcomes allowed Espinosa et al. to propose innovative concepts to study key connections between population-level risk factors and the underlying pathological mechanisms of disease.
Participant data and study design
The research team collected maternal covariates and plasma samples of patients across different international communities. Of the pregnancies on record, a few resulted in preterm births. The researchers obtained details, demographics and characteristics of the pregnancy and measured the maternal proteome, metabolome and lipidome during early and mid-pregnancy.
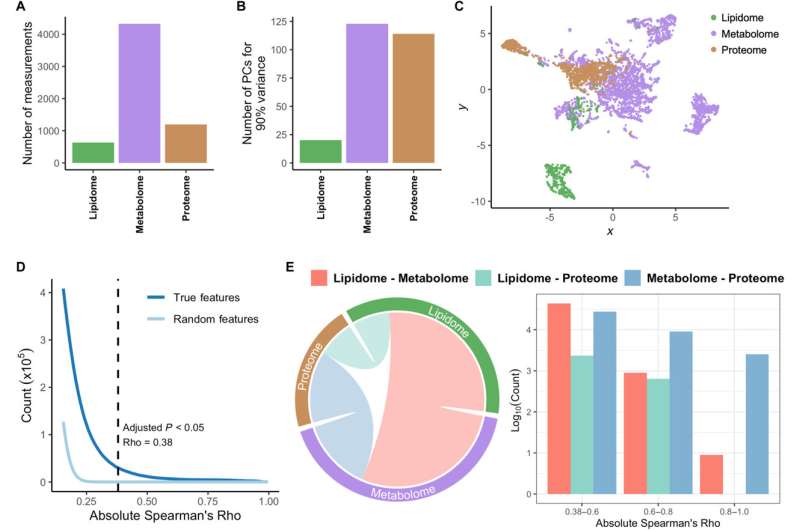
They visualized the combined multi-omics datasets and examined the distribution of significant correlations within intra- and inter-omics during pregnancy to reveal the rigorous orchestration of biological processes during gestation. The resultant pregnancy interactome outlined a strict regulation of biological systems and uncovered their crosstalk during gestation.
This work also highlighted epidemiological characteristics underlying preterm birth, which they cross-validated with a multivariable approach by using a gradient-boosted tree model. The performance of the model showed the existence of a potential risk in the preterm birth population.
They explored several clinical categories to predict preterm birth based on epidemiological features. The analysis described multivariate risk factors associated with the phenomenon to reveal predictive measurements.
Multi-omics modeling to predict premature delivery
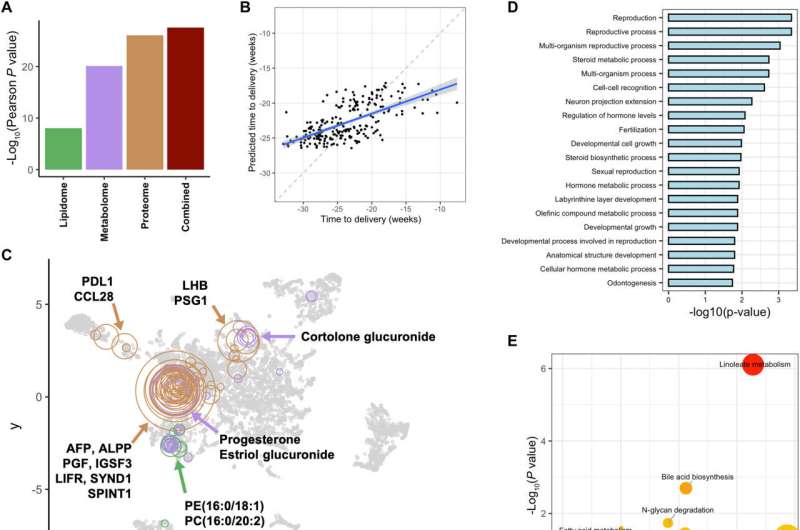
Espinosa et al. conducted multi-omics modeling of the maternal proteome, metabolome and lipidome to predict the time of delivery. The process of pregnancy and the onset of labor are highly coordinated biological events that can be measured via multi-omic profiling. To determine the presence of biological signatures representing the time-frame of delivery, the scientists built a cross-validated XGBoost model.
This model integrated the maternal proteome, metabolome and lipidome at the early stages and mid-pregnancy, while strongly predicting the time of delivery. Of the omics datasets, the proteome matched the highest predictive power of the integrated models, closely followed by the metabolome. The multi-omics profiling robustly captured biological processes underlying early and mid-pregnancy to predict time-of-delivery and show accelerated gestational clocks in women predicted to deliver prematurely.
Age-independent markers of pregnancy memory
The details of a mother's age and obstetric history can impact her risk of preterm birth, although their independent risk contributions remain to be revealed. The multi-omics subset provided predictable variables where maternal age and gravidity correlated significantly, and type 9 collagen showed the strongest association with either covariate.
The scientists noted proteome features of a chemokine ligand (Chemokine ligand 13 CXCL13) and the endothelial nitric oxide synthase to have significant correlations with gravidity, although these biomolecular signatures were not associated with the age of the mother.
This observation suggests an age-independent effect on the maternal proteome, associated with maternal history. The findings highlighted the presence of previously unknown age-independent effects from prior pregnancies that can affect the maternal biology of future pregnancies.
Predictive modeling of maternal body mass index
Maternal body mass index is corelated to preterm birth and results in an increased risk at both ends of the body-mass index spectrum. To examine the impact of this variable on maternal biology, the team-built, cross-validated model with integrated multi-omics data, further validated the strong signature of the body-mass index on the maternal proteome.
Significant features that correlated with this parameter further consisted of metabolism and biomolecule-storage-associated proteins and a cluster of intercorrelated lipid species. The outcomes provided an overview of the epidemiology-associated variables of preterm birth relative to their biological covariates.
Outlook
In this way, Camilo A. Espinosa and colleagues used a large, multinational cohort with widespread data collection to describe epidemiological factors associated with preterm birth. The integrated multi-omics model revealed key biological signatures underlying maternal biological adaptations to pregnancy and preterm birth.
Using a series of bioinformatics models and machine learning algorithms, the team provided fundamental insights to develop biological and socio-economic interventions to attenuate preterm birth across multiple populations.
More information: Camilo A. Espinosa et al, Multiomic signals associated with maternal epidemiological factors contributing to preterm birth in low- and middle-income countries, Science Advances (2023). DOI: 10.1126/sciadv.ade7692
© 2023 Science X Network