June 12, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Neutralizing the side effects of epidermal growth factor inhibitor drugs
Research led by the Hebrew University of Jerusalem, Israel, has found a potential solution to side effects that interfere with cancer treatment. In the paper, "Preventing skin toxicities induced by EGFR inhibitors by topically blocking drug-receptor interactions," published in Science Translational Medicine, the researchers detail how a small molecule SDT-011 reduced the binding affinity of monoclonal antibodies to epidermal growth factor (EGFR) and reactivated EGFR receptor signaling.
EFGR, also called ErbB-1 and Her1, is an enzyme that plays a role in the cell signaling pathway that controls cell division and cell survival. Mutation variants in the EGFR gene can cause the overproduction of EFGR enzymes, accelerating growth in some types of cancer cells. EFGR inhibitors are drugs used to target the activity of this enzyme to slow or stop the growth of cancer cells.
More than a dozen small molecule drugs are available to inhibit EGFR, some permanently, while others are reversible. While a common and effective cancer treatment, off-target toxicities are frequent, including severe acne-like rashes on the face, neck and body, gastrointestinal toxicity and hypomagnesemia, all difficult to treat because another treatment is inducing them.
While ending or reducing cancer growth is the priority, the severity of side effects can result in dose reductions or even discontinuation of treatment. Seeing the need for a solution, the research team sought a toxicity treatment that would not interfere with systemic anticancer therapy.
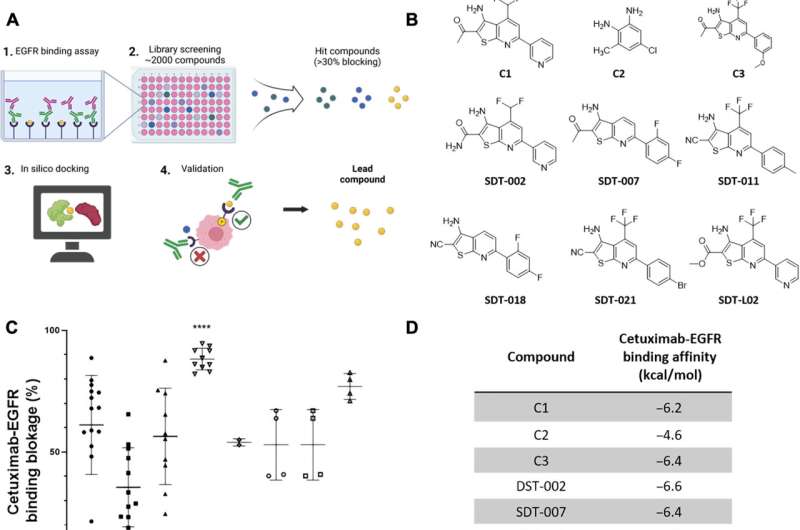
In the current study, researchers screened small molecules to identify those that blocked the binding of the anti-EGFR monoclonal antibodies cetuximab and panitumumab to EGFR. The team identified SDT-011 as the most promising molecule.
SDT-110 reduced the binding affinity of cetuximab to EGFR and reactivated EGFR signaling in vitro and in human skin ex vivo. In addition, topical application of SDT-011 penetrated hair follicles and sebaceous glands. These findings suggest that SDT-011 could potentially treat the on-target skin toxicities experienced by those taking EGFR inhibitors.
In simulations, the docking analysis predicted that SDT-011 interacted with locations on EGFR critical for cetuximab and panitumumab binding. SDT-011 binding to EGFR resulted in a 2,000-fold reduction in the binding affinity of cetuximab to EGFR. SDT-011 was able to reactivate the EGFR pathway in keratinocyte cell lines and cetuximab-treated ex vivo whole human skin.
Importantly, the nanoparticle formulation of SDT-011 used topically blocked the activity of the EGFR inhibitors only at the site of the toxicity without penetrating systemically. The therapeutic inhibitor drugs could continue their anti-cancer activity uninterrupted while SDT-011 neutralized the off-target effects.
More information: Nethanel Friedman et al, Preventing skin toxicities induced by EGFR inhibitors by topically blocking drug-receptor interactions, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abo0684
© 2023 Science X Network