This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A novel testing approach for newly identified autoinflammatory VEXAS syndrome

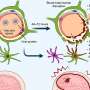
VEXAS syndrome is a severe autoinflammatory disease that results in a spectrum of rheumatologic and hematologic conditions. Mostly affecting men over age 50, VEXAS is caused by somatic mutations in the UBA1 gene of blood cells, which is a gene located in the X chromosome.
Until just a few years ago, patients presenting with features of VEXAS syndrome were not unified under a specific diagnosis. The underlying cause of newly identified VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome was discovered at the National Institutes of Health (NIH) in 2020. A whole exome study investigated patients with an array of adult-onset inflammatory syndromes, discovering a link between previously unrelated complex disorders.
The UBA1 mutation responsible for VEXAS causes the development of painful inflammatory symptoms that can affect the skin, ears, nose, lungs, joints, and vascular system. Hematologic conditions commonly occur, including blood clots, macrocytic anemia, bone marrow abnormalities, and an association with hematologic neoplasms.
"These patients can present on either end of that spectrum with features that look like a complex inflammatory disorder or findings that may suggest a bone marrow cancer," says David Viswanatha, M.D., co-director of the Molecular Hematopathology Laboratory at Mayo Clinic. "Once VEXAS was identified at the NIH, doctors at Mayo Clinic became very interested in this entity and were seeing patients who they suspected had VEXAS, yet we had no test available."
The importance of a proper diagnosis
VEXAS syndrome is estimated to affect approximately 1 in 4,000 men over the age of 50. Because of its recent discovery, limited testing availability, and wide range of symptoms, diagnosing these patients has been a challenge.
"Before the recognition of VEXAS, patients were often labeled with the condition that they seemed to 'fit most,' but often symptoms would not be classical or they would have additional unexplained symptoms that didn't make sense with the labels given," says Matthew Koster, M.D., a rheumatologist at Mayo Clinic. "Often when treating these patients, their symptoms would tend not to respond in the way you would expect a patient with one of the conditions they were believed to have."
Without being able to accurately determine a diagnosis, it's difficult to know the best therapies for treatment. Patients with VEXAS were often cycled between several different immunosuppressive medications.
"When we are able to diagnose a patient with VEXAS, we are able to move towards medications that have been shown to have better benefit and avoid medications that have been trialed and found to be ineffective," says Dr. Koster. "It also allows us to determine severity and extent of disease and determine what type of therapies might be more suitable for controlling inflammation, which can range from steroids to immunosuppressive medications, to hematologic drugs, to bone marrow transplant."
A new test for a newly identified disease
With a relentless commitment to innovation and a drive to improve patient care, Mayo Clinic Laboratories' medical directors and staff quickly worked on developing a new test to detect the UBA1 mutations responsible for VEXAS.
Within six months of NIH's discovery, the test was added to the latest MayoComplete next-generation sequencing for myeloid neoplasms panel (Mayo ID: NGSHM). This addition allows doctors to detect mutation of the UBA1 gene, along with 46 other genes, while evaluating patients with known or suspected hematologic cancers of myeloid origin.
The addition to the MayoComplete panel was an important first step in providing a UBA1 testing option to doctors and their patients. The next step was developing a single gene assay to allow doctors to test specifically for UBA1 mutations that could be used to screen patients for VEXAS syndrome.
"When looking at what methodology would provide the best option for clinical care, we decided on the development of a droplet digital polymerase chain reaction (ddPCR) test," says Dr. Viswanatha. "There are other types of molecular diagnostic assays that would have been faster and simpler to develop, but ultimately we wanted a limit of detection threshold of 0.5% for maximum sensitivity and high accuracy."
An award-winning development
Aimee Boerger, a senior developer in the Molecular Hematopathology Laboratory at Mayo Clinic, teamed up with Dragana Milosevic, principal developer in the Genomics Laboratory at Mayo Clinic, to swiftly begin designing the new ddPCR test. It was designed to use peripheral blood or bone marrow aspirate samples and investigate single point mutation changes at seven targeted loci in the DNA of the UBA1 gene.
"The ddPCR assay is meant to be more specific," says Aimee. "It's for doctors to order for patients who are showing the symptoms of VEXAS, and when doctors have a high suspicion of a VEXAS diagnosis. This offers a more sensitive, more targeted, and hopefully more cost-effective test option for patients. It's also more convenient for a patient to have blood drawn as opposed to needing a more invasive specimen."
Because of the quantifiable process involved in a ddPCR test, it offers high sensitivity, which is a key differentiator from other methodologies.
"Regular PCR is quite sensitive, but with ddPCR it partitions the sample into 20,000 tiny droplets, and then you carry out the polymerase chain reaction in each of those 20,000 droplets," says Aimee. "So, we're able to get that sensitivity level down to 0.5% VAF (variant allele frequency) and achieve absolute quantification. In addition, there are seven targets as part of this assay to detect any of the seven mutations of the UBA1 gene that can cause VEXAS syndrome."
Using ddPCR to detect UBA1 gene mutations is a novel approach—and a feat that was marked by Aimee receiving the Technologist Poster Award at the Association for Molecular Pathology annual meeting in 2022.
"This was the first test that I got to design and develop, with Dragana's help, from the ground up," says Aimee. "Designing an assay to detect that many targets was a really rewarding challenge and eye-opening experience. It involved working across divisions and sharing best practices."
"We were able to get from the ideation of the test to test implementation within six months. We are fortunate to have great people, methods, data, and technology, and as discoveries are made at Mayo Clinic or at other institutions like NIH, we have the expertise to rapidly develop new tests and health care services."
Aimee is hopeful that this test, which will soon be available to ordering physicians through Mayo Clinic Laboratories, will make a significant impact on advancing patient care.
"It is super rewarding to finally be able to give previously misdiagnosed patients the answers they have been searching for," says Aimee. "I hope by helping to identify this disease we can bring more attention to it, determine the prevalence, and get patients enrolled in clinical trials to help get them the care that they need."
Offering hope for patients with VEXAS
With the UBA1 assay added to the MayoComplete panel (Mayo ID: NGSHM) and the creation of the new ddPCR test, patients with VEXAS have more opportunities for an accurate diagnosis.
"If a patient comes into a clinic, for instance, having VEXAS-like symptoms, a rheumatologist can send the patient for a blood draw and order UBA1 testing," says Dr. Viswanatha. "On the other hand, many of these patients may initially see a hematologist, who may suspect myelodysplasia and order a bone marrow biopsy to investigate for a myeloid malignancy. We put the UBA1 gene on the MayoComplete panel to cover both scenarios. We're trying to anticipate the point of entry where patients may be diagnostically encountered and how the testing may be ordered in those situations."
As more health care providers become aware of this condition, testing will increase, and more patients will be diagnosed. However, there is still much research needed on VEXAS and its treatment options. Prednisone has been shown to work well as a treatment, but at doses that are not sustainable long-term because of the development of adverse steroid-associated side effects.
"There is currently no approved medication that is known to work in all patients with VEXAS," says Dr. Koster. "Some medications seem to have at least a partial impact on the inflammatory process. Treatment trials are being developed to see if more effective steroid-sparing agents can be found to help control the disease. Bone marrow transplant is being explored, and this has been done at Mayo Clinic in Rochester, Minnesota, which has the highest volume of bone marrow transplants for VEXAS among centers in the United States."
Mayo Clinic physicians and scientists continue to actively engage in research on VEXAS, including biorepository studies and translational research, to increase the knowledge around drugs and bone marrow transplant in this condition. Several researchers are also part of an international consortium with the goal of creating more uniform standards and treatments for VEXAS.
"This is bigger than just what we're doing at Mayo Clinic," says Dr. Viswanatha. "We're able to collaborate and hopefully contribute to patient care in a significant way, helping to establish standards for patients all over the world that may end up being diagnosed with this disease."