This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study sheds light on origins, changeability of blood stem cells in humans
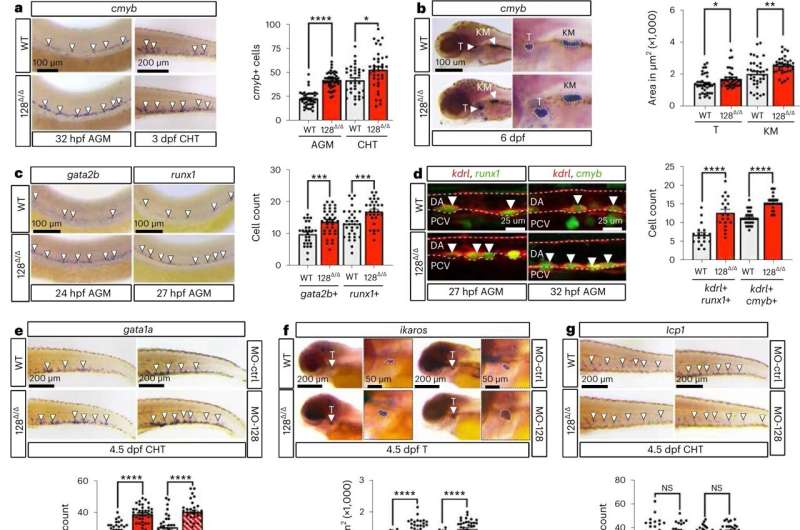
All humans have a diverse set of blood stem cell types which dictate the composition and function of our blood and immune cells and ultimately help govern overall health. Older people tend to lose this diversity of blood stem cells, which can make them more susceptible to blood cancers, cardiovascular diseases, and all-cause mortality. But exactly when and how this diverse group of stem cells first arise has been unclear.
A group of researchers at Yale School of Medicine has found that levels of diversity of blood stem cells are determined during the development of the embryo, they report July 17 in the journal Nature Cell Biology. They also found that stem cell levels can be manipulated during childhood, suggesting ways that blood composition might be improved, and overall health monitored throughout life.
"The findings are quite powerful," said Stefania Nicoli, associate professor in internal medicine and genetics at Yale School of Medicine and senior author of the paper.
Scientists had thought that this diversity of stem cells arose later in development with the formation of bone marrow. However, Joey Ghersi, a postdoctoral associate in Nicoli's lab, working in collaboration with Christopher Sturgeon at Mount Sinai School of Medicine, showed that different types of blood stem cells were determined very early in development.
"And each of us maintain this level of diversity through adulthood," Nicoli said. "This varying level of blood stem cell diversity can be 'programmed' and may help to change susceptibility to cardiovascular and immune diseases."
For example, Nicoli and colleagues manipulated levels of one microRNA within embryonic endothelial cells that line blood vessels and enhanced production of blood stem cells that increase the level of red blood- and T-cells in zebrafish and human pluripotent stem cells.
"The zebrafish with manipulated blood stem cells remained healthy through life," Nicoli said.
As more is learned about how stem cell populations affect health, the ability to manipulate those populations will become more important in health care, Nicoli predicted.
More information: Ghersi, J.J., et al. Haematopoietic stem and progenitor cell heterogeneity is inherited from the embryonic endothelium. Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01187-9 www.nature.com/articles/s41556-023-01187-9


















