This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New data on COVID-19 vaccine response and clinical outcomes in patients with impaired immune systems
Researchers and clinicians from the University of Sheffield and Sheffield Teaching Hospitals NHS Foundation Trust are part of the ongoing OCTAVE (Observational Cohort Trial-T-cells Antibodies and Vaccine Efficacy in SARS-CoV-2) trial, which is led by the Universities of Glasgow, Birmingham and Oxford and a consortium of leading UK institutions.
Updated data from the OCTAVE study show, for the first time, the real-world vaccine responses and infection outcomes in clinically at-risk patients with a range of immunocompromised or immunosuppressed conditions.
Published in Nature Medicine, the latest report contains important new data on infection rates, disease severity and deaths in the patient groups, who were studied up to one year after their first vaccination.
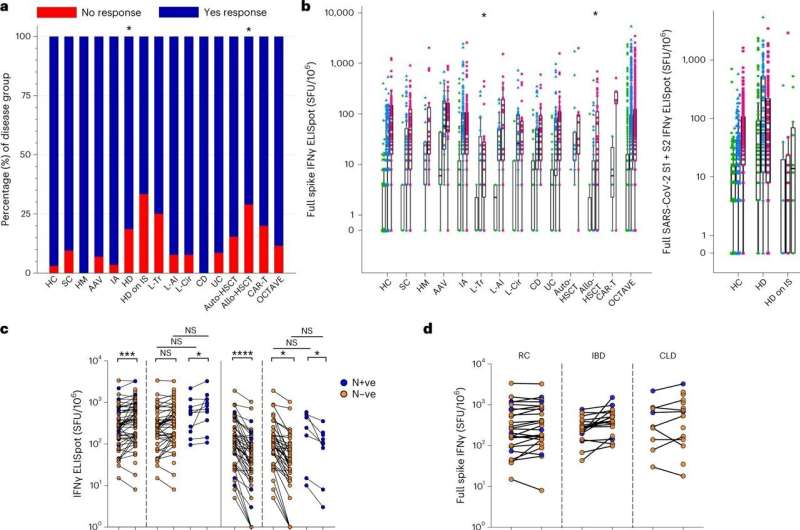
Preliminary data from the OCTAVE study in August 2021 showed that a significant proportion of clinically at-risk patients with immunocompromised or immunosuppressed conditions, mounted a low, or undetectable, immune response after two doses of the same SARS-CoV-2 vaccine. Now, in new peer-reviewed data, researchers are able to share the real-world infection outcomes for this clinically at-risk group.
Data from the study cover the time period from 2021 to mid-2022, and include patients infected with the alpha, delta and omicron strains of SARS-CoV-2. The data do not estimate the impact of third and fourth vaccinations, which have since been offered to patients in the groups studied.
These new data show that, while in most at-risk patient groups the overall COVID-19 infection rates were low, the risk of severity and death from SARS-CoV-2 was high in a sub-group of conditions, despite vaccination. This was particularly the case during the delta wave. Furthermore, the data show that while omicron, now the dominant SARS-CoV-2 strain worldwide, saw a rise of infection rate among at-risk patients, fewer of them became severely unwell or died.
OCTAVE is a multi-center, UK-wide trial. It was set up in the middle of the COVID-19 pandemic to evaluate, in real time, the immune responses following COVID-19 vaccination in patients with immune-mediated inflammatory diseases such as cancer, inflammatory arthritis, diseases of the kidney or liver, or patients who are having a stem cell transplant.
Previously, preliminary data released from OCTAVE in 2021 showed that while most patients mounted a successful immune response after two doses of the vaccine, some patients with certain immunosuppressed conditions mounted a low, or undetectable, immune response.
The study found that overall 12% of patients on the trial failed to develop antibodies, with an additional 27% only generating low levels of antibodies. Some patients also failed to generate adequate T cell responses after vaccination. Vaccine failure rates were higher in some subgroups including patients with ANCA-associated vasculitis on the drug rituximab, patients receiving hemodialysis and also on immunosuppressive therapy, and patients who were solid organ transplant recipients.
The study used a variety of state-of-the-art immune tests performed on blood samples taken before and/or after COVID-19 vaccination, as well as infection and severity data on patients to better understand the real-world impact of a low vaccination response in these patient groups.
In the UK, more than 60% of people aged over 65 were known to have one or more chronic disease in 2019, while more than 12 million people aged 18–65 were living with a chronic condition lasting longer than 12 months. Government estimates suggest that around 500,000 people have an immune suppressive disease in the UK—a significant group which may be more at risk of severe COVID-19 infection if not fully protected by vaccination.
Thushan de Silva, Professor of Infectious Diseases at the University of Sheffield and Honorary Consultant Physician in Infectious Diseases at Sheffield Teaching Hospitals NHS Trust, added, "The OCTAVE study has demonstrated which patients may remain at greater risk of COVID-19 despite vaccination and where we need to focus further efforts to find ways to protect the most vulnerable groups in society."
John Snowden, Director of Blood and Marrow Transplantation Program at Sheffield Teaching Hospitals NHS Trust and Honorary Professor at the University of Sheffield, said, "Patients who have received various forms of treatment for blood cancers, including bone marrow transplantation and CAR-T therapy, are particularly susceptible to developing severe complications of COVID-19 infection, yet many respond poorly to vaccination.
"The OCTAVE study has provided major insights into how we can best identify the most vulnerable patients and protect them as possible against severe complications.
"Working with many national teams, the Sheffield team led the section of the OCTAVE study focussing on bone marrow transplant and CAR-T patients and was the largest recruiter of patients into this high-risk cohort. We sincerely thank all patients and staff involved in this great achievement."
The OCTAVE trial is one of the largest studies in the world so far into post-SARS-CoV-2 vaccination in immunocompromised patients.
Professor Iain McInnes, lead of the OCTAVE trial, and Vice Principal and Head of the College of MVLS at the University of Glasgow, said, "The OCTAVE study has provided vital insights into the effectiveness of SARS-CoV-2 directed vaccines in some of our most vulnerable patient groups."
"The study's key strengths include identifying the small number of patients who may not respond to the vaccines, enabling healthcare providers and policy makers to make the best decisions to protect these groups of people. Importantly the study has also been able to reassure us that the majority of our immunocompromised patients in the UK have been protected from severe COVID-19 by the vaccination program."
Professor Pamela Kearns, Director of the Cancer Research UK Clinical Trials Unit at the University of Birmingham said, "OCTAVE provides an important understanding of how vaccines provided protection for many groups of the most clinically vulnerable, and how the variants of the disease affected how effective they were.
"We can see that there are areas of particular concern where vaccines didn't adequately protect against COVID-19, including some patients with renal diseases and some inflammatory conditions.
"We're immensely grateful for all those participants and each of the centers that took part to give us the clearest picture yet of how the most clinically vulnerable were protected, and where there are areas of weakness that need to be addressed."
Professor Eleanor Barnes, Professor of Hepatology and Experimental Medicine at Nuffield Department of Medicine, University of Oxford, said, "Importantly, both the T cells and Antibody responses to vaccines were shown to protect against severe disease in immunocompromised patients."
The OCTAVE findings in detail
- There were 20 hospital sites across the UK who enrolled 2,686 patients with reduced function of their immune systems to the trial.
- Overall, 474 patients in the study became ill with COVID-19 up to one year after the date of their first vaccination. 111 of these infections were identified as either alpha (1) or delta (110) variants and 336 were identified as omicron. Of the 48 people admitted to hospital because of a COVID-19 infection, 15 people died from the disease.
- Most infections (76%) occurred more than six months after the second vaccination and were in patients with a kidney transplant, inflammatory arthritis and Crohn's Disease. The majority of these infections were also first-time SARS-CoV-2 infections. Infections occurring within six months of the second vaccination were not more severe than those reported six months or longer post second vaccination.
- Most infections (90%) were mild in severity, including some asymptomatic cases. Severe cases requiring hospitalization or death was reported in 9.8% of infections and occurred predominantly in patients with renal disease. Patients infected in the delta wave were more likely to have serious infection than those infected in the omicron wave.
- In patients who did not mount a successful immune response to two COVID-19 vaccinations, the rate of infection was higher when compared to those in the study that did mount an immune response after vaccination.
- In some disease groups, the overall infection rates were low—possibly because of continued shielding at the time—but the proportion of severe cases within these groups were high. This was most notable in patients with ANCA Associated Vasculitis on rituximab, auto and allogeneic stem cell transplant and CART-T cell treated patients.
- Low rates of severe disease were reported in patients with Crohn's disease and ulcerative colitis.
More information: Eleanor Barnes et al, SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease, Nature Medicine (2023). DOI: 10.1038/s41591-023-02414-4