This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves topical treatment for molluscum contagiosum
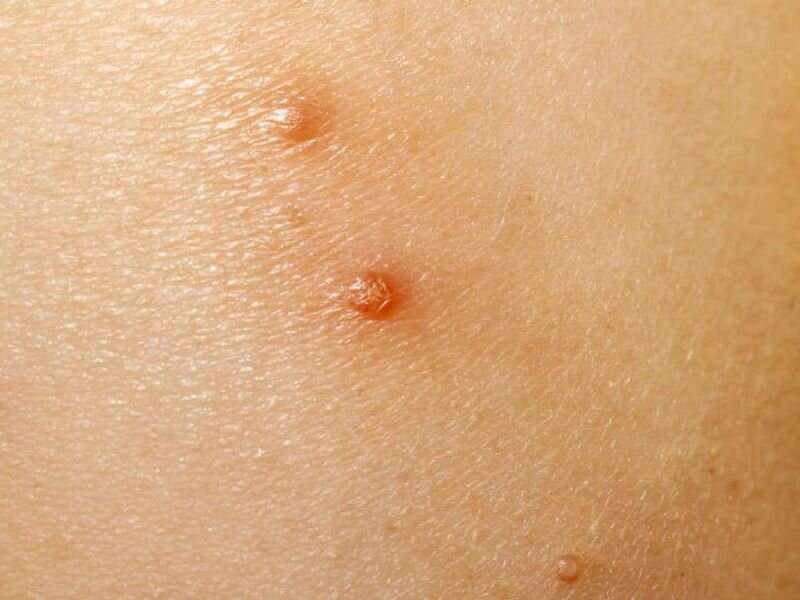
The U.S. Food and Drug Administration has approved the topical solution Ycanth (cantharidin) for the treatment of molluscum contagiosum (molluscum) in adult and pediatric patients (aged 2 years and older).
Ycanth uses a single-use applicator to deliver a good manufacturing practices-controlled formulation of cantharidin (0.7 percent), allowing for precise topical dosing and targeted administration.
The approval was based on two identical phase 3 trials (CAMP-1 and CAMP-2). Both trials showed that a clinically and statistically significant number of patients treated with VP-102 (Ycanth) met the primary end point of complete clearance of all treatable molluscum lesions (CAMP-1: 46 percent of participants treated with VP-102 achieved complete clearance versus 18 percent in the vehicle group; CAMP-2: 54 versus 13 percent, respectively). Statistically significantly higher rates of complete clearance were seen across all body regions, including the most sensitive areas, and across all ages.
Local skin reactions at the application site occurred in most Ycanth-treated participants (97 percent), including vesiculation, pruritus, pain, discoloration, and erythema. No serious adverse reactions were reported, with discontinuation rates due to an adverse reaction in 2.3 percent of the Ycanth group and 0.5 percent of the vehicle-treated group.
"Since molluscum spreads through skin-to-skin contact and the sharing of contaminated objects with its viral lesions, a topical treatment with precise administration is essential towards preventing further transmission," Ted White, Verrica president and CEO, said in a statement. "Based on the results from Verrica's clinical trials, the FDA found that Ycanth is safe and effective for patients as young as 2 years old, providing an important treatment option for patients and caregivers struggling with this disease."
Approval of Ycanth was granted to Verrica Pharmaceuticals.
More information: More Information
Copyright © 2023 HealthDay. All rights reserved.