This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Substance use linked to long-lasting brain changes, cognitive decline
An estimated 50 million individuals in the United States struggle with the challenges of cocaine or alcohol use disorders, according to the National Institutes of Health (NIH). Beyond the well-documented health risks, addiction to these substances detrimentally affects our cognitive flexibility, which is the ability to adapt and switch between different tasks or strategies. Although previous research has hinted at this connection, the underlying reasons for this cognitive impairment remain elusive.
Cognitive flexibility is a crucial element in various domains of our life, including academic achievement, employment success and transitioning into adulthood. As we age, this flexibility plays an important role in mitigating cognitive decline. A deficiency in cognitive flexibility, however, is linked to academic deficits and a lower quality of life.
A study led by Dr. Jun Wang, associate professor in the Department of Neuroscience and Experimental Therapeutics at the Texas A&M University School of Medicine, provides new insight into the damaging impact that chronic cocaine or alcohol use has on cognitive flexibility.
The research, published in the journal of Nature Communications, emphasizes the role of the local inhibitory brain circuit in mediating the negative effects of substance use on cognitive flexibility.
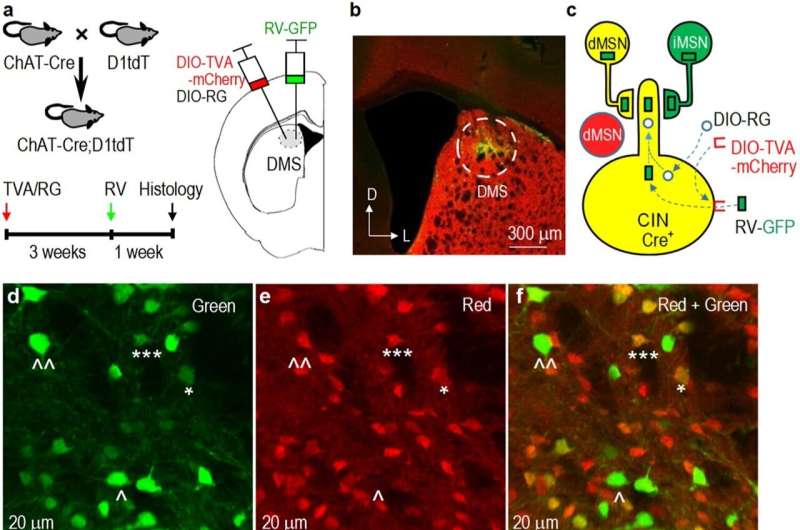
Substance use influences a specific group of neurons called striatal direct-pathway medium spiny neurons (dMSNs), with projections to a part of the brain known as the substantia nigra pars reticulata (SNr). Conversely, cognitive flexibility is facilitated by striatal cholinergic interneurons (CINs), which receive potent inhibitory signals from the striatum.
"Our hypothesis was that increased dMSN activity from substance use inhibits CINs, leading to a reduction in cognitive flexibility," Wang said.
"Our research confirms that substance use induces long-lasting changes in the inhibitory communication between dMSNs and CINs, consequently dampening cognitive flexibility. Furthermore, the dMSN-to-SNr brain circuit reinforces drug and alcohol use, while the associated collateral dMSN-to-CIN pathway hinders cognitive flexibility. Thus, our study provides new insights into the brain circuitry involved in the impairment of cognitive flexibility due to substance use."
More information: Himanshu Gangal et al, Drug reinforcement impairs cognitive flexibility by inhibiting striatal cholinergic neurons, Nature Communications (2023). DOI: 10.1038/s41467-023-39623-x