This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Anakinra is safe but does not reduce complications of acute myocarditis, research suggests
The largest randomized controlled trial of patients with acute myocarditis has found that anakinra is safe but does not reduce complications. The late breaking research presented in a Hot Line session today, August 28, at ESC Congress 2023.
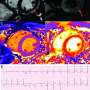
Acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias and death. The disease can occur in individuals of all ages, but is most frequent in the young.
There are no specific therapies, and patients are generally treated with beta-blockers, ACE inhibitors and sometimes steroids, in accordance with an ESC expert consensus. Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. While anakinra is effective in pericarditis, there are only case reports of successfully treated acute myocarditis.
ARAMIS was the largest randomized controlled trial of acute myocarditis and the first to evaluate inhibition of the interleukin-1β innate immune pathway in these patients. The trial enrolled hospitalized, symptomatic patients with increased cardiac troponin and acute myocarditis diagnosed using cardiac magnetic resonance (CMR) imaging.
Patients were randomly allocated in a 1:1 ratio within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard of care treatments, including an ACE inhibitor for at least one month. The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction <50%, and ventricular arrythmias) within 28 days post-discharge.
The study included 120 patients from six academic centers in France. A total of 117 patients were included in the intention-to-treat analysis. Consistent with prior data, the median age of participants was 28 years and nearly 90% were men.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients. There was no difference in the number of days free of any myocarditis complications between the two arms, with a median (quartile 1, quartile 3) of 30 days (30, 32) for anakinra versus 31 days (30, 32) for placebo, for a difference of 0.0 (95% confidence interval -1.0 to 0.0, p=0.168).
The safety endpoint was the number of serious adverse events within 28 days post-discharge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days post-discharge were reported in both arms.
Principal investigator Dr. Mathieu Kerneis of Pitié Salpetrière APHP University Hospital, Paris, France said, "ARAMIS enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications. A short course of anakinra did not increase the number of days free of myocarditis complications. There was no safety issue with anakinra administered during the acute phase of myocarditis diagnosed without endomyocardial biopsy, and therefore no proof of viral status.
"Further randomized controlled trials are needed to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at high risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications."