This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
Atezolizumab monotherapy beneficial for non-small cell lung cancer ineligible for platinum-based chemo
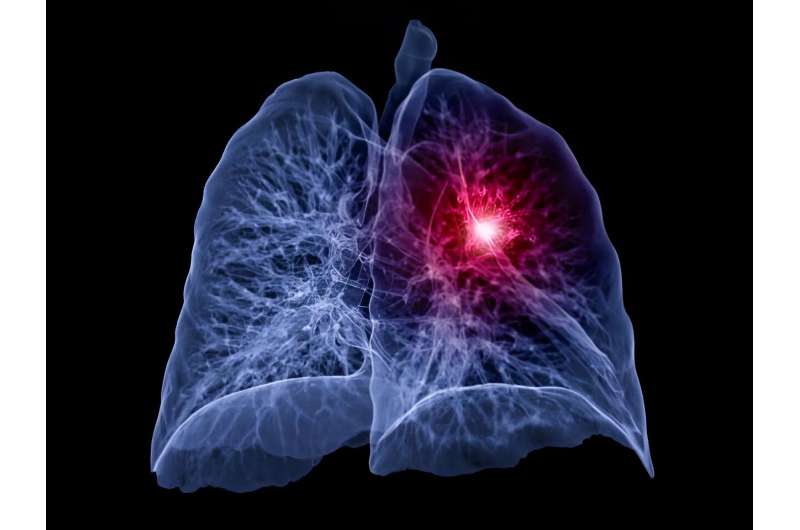
For patients with non-small cell lung cancer (NSCLC) ineligible for platinum-based chemotherapy, first-line atezolizumab monotherapy is associated with improved overall survival compared to single-agent chemotherapy, according to a study published online Aug. 5 in The Lancet.
Siow Ming Lee, M.B.B.S., Ph.D., from the University College London, and colleagues conducted a randomized controlled trial at 91 sites in 23 countries to compare the efficacy and safety of first-line atezolizumab monotherapy with single-agent chemotherapy among patients with stage IIIB or IV NSCLC in whom platinum-doublet chemotherapy was deemed unsuitable. A total of 453 patients were enrolled and randomly assigned to atezolizumab or chemotherapy (302 and 151 patients, respectively).
The researchers found that compared with chemotherapy, atezolizumab improved overall survival (median overall survival, 10.3 versus 9.2 months; stratified hazard ratio, 0.78), with a two-year survival rate of 24 percent with atezolizumab and 12 percent with chemotherapy. Atezolizumab was associated with stabilization or improvement of patient-reported health-related quality-of-life functioning scales and symptoms compared with chemotherapy, and with fewer grade 3 to 4 treatment-related adverse events (16 versus 33 percent) and treatment-related deaths (1 versus 3 percent).
"First-line atezolizumab was associated with improved survival and a doubling of the two-year survival rate, despite over 50 percent of patients in the chemotherapy arm who were still alive at two years receiving subsequent immunotherapy," the authors write.
Several authors disclosed ties to pharmaceutical companies, including F. Hoffmann-La Roche and Genentech, who funded the study; Genentech is the manufacturer of atezolizumab.
More information: Siow Ming Lee et al, First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study, The Lancet (2023). DOI: 10.1016/S0140-6736(23)00774-2
Hazel O'Sullivan et al, Atezolizumab in patients with advanced non-small-cell lung cancer who are platinum-doublet ineligible, The Lancet (2023). DOI: 10.1016/S0140-6736(23)00807-3
Copyright © 2023 HealthDay. All rights reserved.