This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research sheds light on how breast cancer might develop after pregnancy
A study observing changes in healthy breast cells may explain why breast cancer might develop after pregnancy. The cell-based study has helped begin to disentangle the complex relationship between genetic mutations, pregnancy and breast cancer risk. The research has been published in the journal Nature Communications.
Researchers from Imperial College London examined healthy breast cells from 29 women who had given birth at different ages and women who did not have any children, to look at genetic mutations and how cells divide.
It is the first time such a study has been performed and provides important clues as to what happens when breast cells turn cancerous. Crucially, it may explain why women who have their first child later in life appear to have a higher long-term risk of breast cancer compared to women who have children earlier.
Lead author of the study, Dr. Biancastella Cereser, from Imperial's Department of Surgery and Cancer, says, "In recent decades, women have begun having children later because of societal changes and personal preferences. Previous research has found that this is associated with a heightened breast cancer risk."
"Our own research delves into the genetic mysteries that govern this risk. We found that the human breast, like other organs, accumulates mutations with age—but also that pregnancy has an additional effect meaning that older first-time mothers might have a higher chance of developing harmful changes in their breast cells compared to other women."
Breast cancer risks
A woman's risk of developing breast cancer is influenced by pregnancy—but the relationship is complex.
Several studies report that young first-time mothers (generally considered to be those under 24 years at first pregnancy) have about a 20%–35% lower risk of developing breast cancer in the long-term when compared with women who do not have any children.
However, the risk of breast cancer then progressively increases for mothers who have their first child after the age of 24—with a 5% increase in risk for every five years.
Breast tissue changes
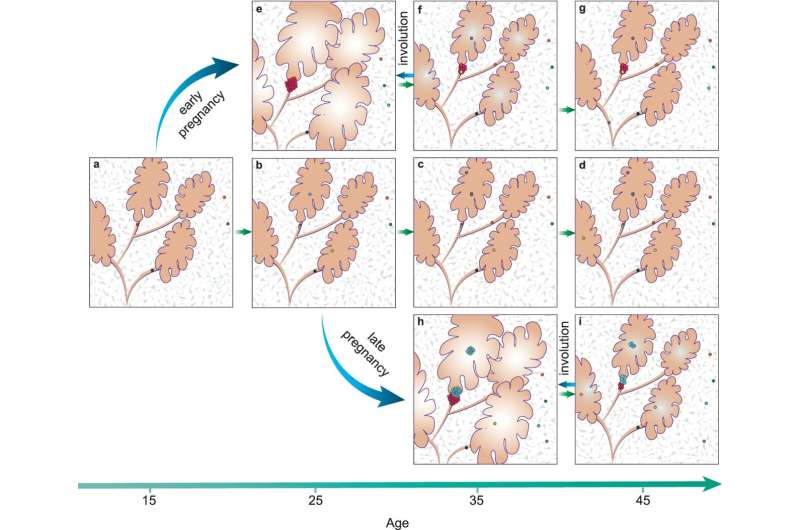
In the latest study, Dr. Cereser and team examined in detail the cellular and genetic changes that take place in normal, healthy breast tissue in different groups of women: first-time mothers under 25 years; first-time mothers between 35 and 55; and women with no children (aged between 25 and 53).
They sequenced the entire genomes of 29 frozen healthy breast tissues from donors with no previous use of hormonal contraception and without inherited mutations in BRCA1 or BRCA2 genes (which substantially increase the risk of breast cancer).
They found that the healthy breast accumulates mutations with age, at rate of about 15 mutations a year in the epithelium tissue (the cell type from which breast cancer typically arises). The majority of these mutations have no negative effect at all and were not in genes known to be associated with cancer—as would be expected in healthy tissue.
The researchers were also able to look at groups of cells with the same genetic profile, called clonal patches. These arise when a mutated cell shares its mutation with its daughter cells, expanding the population of mutated cells. Importantly, breast tissue from first-time mothers aged between 35 and 55 years had significantly more, and larger, clonal patches.
Based on this evidence, Dr. Cereser believes that as women age their breast cells naturally accumulate more mutations, and given more time there's a greater chance that one or more of those mutations occurs in genes that are associated with cancer (also called "driver mutations"). This might not be enough to cause cancer by itself. But pregnancy could provide a "double whammy," because it induces a rapid expansion of breast cells, in preparation for breast feeding. If cells harboring driver mutations replicate and expand, they could have a competitive advantage over neighboring non-mutated cells, potentially leading to a runaway effect, and ultimately creating a cancerous tumor.
Women's health gap
However, she cautions that there is still much more research to be done, and the study cannot answer key questions, including the impact of breastfeeding, age at first period and menopause on breast cancer risk.
Commenting on the findings, Dr. Cereser concludes, "Nobody has looked at the entire genome of the healthy breast before, let alone differentiating into groups of women who had given birth at different ages, as well as women who did not have any children. I hope this study could be used as a resource for researchers looking at the genetics of healthy tissue in general, aside from the breast, and of course other breast cancer researchers, who could use the dataset of mutations we found as a 'reference.'"
More information: Biancastella Cereser et al, The mutational landscape of the adult healthy parous and nulliparous human breast, Nature Communications (2023). DOI: 10.1038/s41467-023-40608-z