This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A new communication medium between cardiomyocytes and fibroblasts
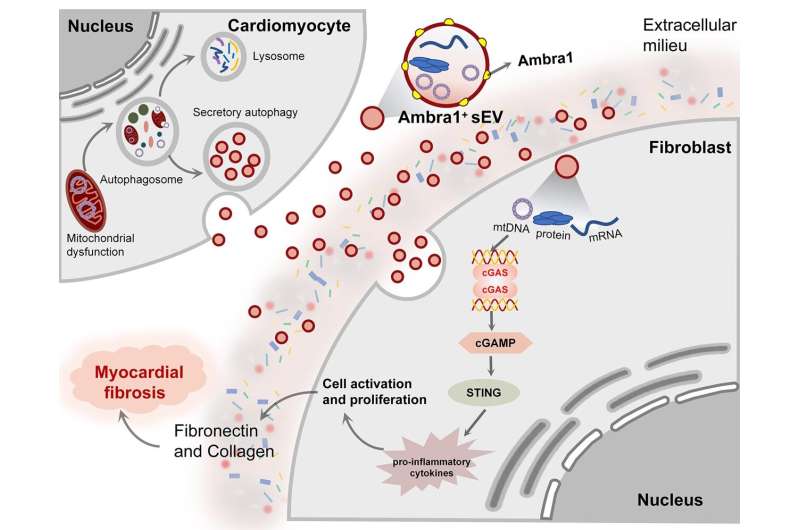
Myocardial fibrosis is major pathological outcome after myocardial ischemia/reperfusion (MI/R) injury and a major risk factor for heart failure. MI/R injury induces cardiomyocyte damage or even death, which in turn stimulates fibroblast activation and fibrosis, but the intercellular communication mechanism remains unknown.
Recent studies have shown that small extracellular vesicles (sEVs) significantly contribute to intercellular communication. Whether and how small extracellular vesicle might mediate post-MI/R cardiomyocyte/fibroblasts communication remain unknown. The role of altered communication between damaged cardiomyocytes and fibroblast activation in post-MI/R cardiac remodeling remains controversial.
A new study was led by Prof. Heng Ma (School of Basic Medicine, Fourth Military Medical University), Prof. Alex Chia Yu Chang (Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine), and Prof. Lu Yu (Xijing Hospital, Fourth Military Medical University).
This study demonstrates that sEVs derived from cardiomyocyte (Myo-sEVs) carry mitochondrial components, which enters fibroblasts to initiate myocardial fibrosis. Based on bioinformatics screening and experimental verification, Ambra1 was found to be a critical component of these sEV and might be a new marker for Myo-sEVs.
Interestingly, release of Ambra1+-Myo-sEVs was caused by secretory rather than canonical autophagy after MI/R injury and thereby escaped degradation. In ischemic and peripheral areas, Ambra1+-Myo-sEVs were internalized by fibroblasts, and the delivered mtDNA components to activate the fibroblast cGAS-STING pathway to promote fibroblast activation and proliferation.
In addition, the data show that Ambra1 is expressed on the EV surface and cardiac-specific Ambra1 down regulation inhibits the Ambra1+-Myo-sEVs release and fibroblast uptake, effectively inhibiting ischemic myocardial fibrosis. This finding newly provides the evidence that myocardial secretory autophagy plays a role in intercellular communication during cardiac fibrosis. Ambra1 is a newly characterized molecule with bioactivity and might be a marker for Myo-sEVs, providing new therapeutic targets for cardiac remodeling.
First, Chan Zhang et al. examined the extent of myocardial fibrosis and molecular signatures after MI/R. They found that MI/R leads to pathological fibrosis, cGAS-STING pathway activation and impaired autophagic degradation in the infarct area. In addition, MI/R significantly affected the number, protein content, and size of Myo-sEVs. Myo-sEVsI/R formation and release may be driven by the autophagic functional status.
Next, the molecular tags/signals identifying the secreted substrates in Myo-sEVsI/R were further analyzed. The result suggested that Ambra1 combined with specific marker of the autophagosomes (LC3) can be used to identify Myo-sEVsI/R.
Subsequently, after demonstrating that Ambra1+ EVs are cardiac-specific extracellular vesicles of autophagic origin, the researchers further evaluated whether the Ambra1+Myo-sEVI/Rcan mediate the communication between cardiomyocytes and fibroblasts.
Both in vitro and in vivo studies indicate that Ambra1+Myo-sEVI/R, which carries mitochondrial components and mtDNA, can be internalized to promote pathological proliferation of fibroblasts through activation of cGAS-STING pathway. Finally, the team found that pathological activation of cardiac fibroblasts after MI/R could be attenuated by myocardial-specific Ambra1 knockdown.
The present study demonstrates a novel myocardial mitochondrial efflux mode, i.e., autophagosome-derived Ambra1+Myo-sEVsI/R efflux of mitochondrial components.
The study is published in the journal Science Bulletin.
More information: Chan Zhang et al, Intercellular mitochondrial component transfer triggers ischemic cardiac fibrosis, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.07.030