This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
DNA damage-induced senescence model in osteoarthritic chondrocytes
A new research paper titled "Development of a DNA damage-induced senescence model in osteoarthritic chondrocytes" has been published in Aging.
Senescent cells (SnCs) have been described to accumulate in osteoarthritis (OA) joint tissues in response to injury, thereby participating in OA development and progression. However, clinical therapeutic approaches targeting SnCs using senolysis, although promising in preclinical OA models, have not yet proven their efficacy in patients with knee OA. This pitfall may be due to the lack of understanding of the mechanisms underlying chondrocyte senescence.
In their new study, researchers Mélina Georget, Anaïs Defois, Romain Guiho, Nina Bon, Sophie Allain, Cécile Boyer, Boris Halgand, Denis Waast, Gaël Grimandi, Alban Fouasson-Chailloux, Jérôme Guicheux, and Claire Vinatier from Nantes Université aimed to generate models of chondrocyte senescence.
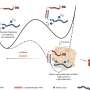
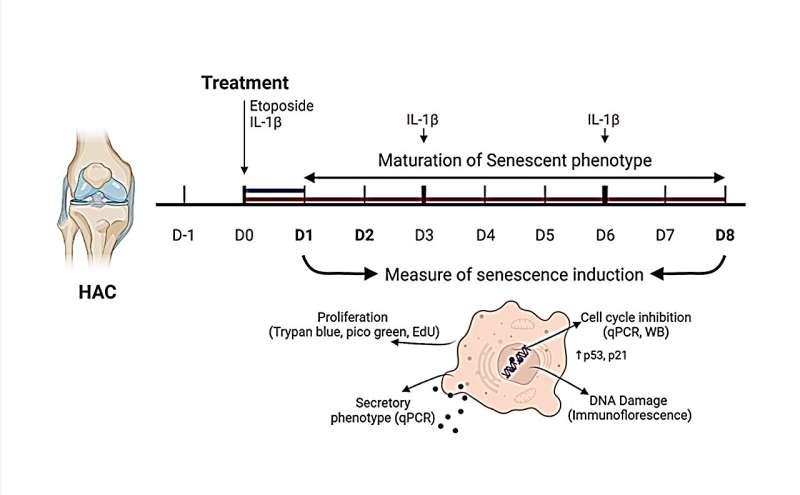
The researchers explain, "In this context, our study aims to develop in vitro models of chondrocyte senescence by investigating the ability of etoposide and IL-1β treatments to produce a reliable chondrocyte senescent model."
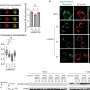
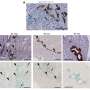
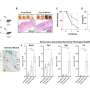
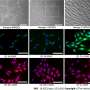
This study used etoposide to induce DNA damage-related senescence, or chronic exposure to IL-1β to entail inflammation-related senescence in human OA chondrocytes. Several hallmarks of cellular senescence, such as cell cycle arrest, expression of cyclin-dependent kinase inhibitors, DNA damages, and senescence-associated secretory profile were evaluated.
Chronic exposure to IL-1β induces only partial expression of senescence markers and does not allow us to conclude on its ability to induce senescence in chondrocytes. On the other hand, etoposide treatment reliably induces DNA damage-related senescence in human articular chondrocytes evidenced by loss of proliferative capacity, DNA damage accumulation, and expression of some SASP components.
"Etoposide-induced senescence model may help investigate the initiation of cellular senescence in chondrocytes, and provide a useful model to develop therapeutic approaches to target senescence in OA," the researchers conclude.
More information: Mélina Georget et al, Development of a DNA damage-induced senescence model in osteoarthritic chondrocytes, Aging (2023). DOI: 10.18632/aging.204881