This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Q&A: How medicine can achieve more diversity in clinical trials—an expert's perspective

The phrase "representative sample" is common parlance in scientific studies—and it's commonly used these days to talk about a lack of racial and ethnic representation in clinical trials.
Researchers tend to consider factors such as age, education level and marital status when selecting a small sample that reflects the demographics of a larger population. Unfortunately, study designers often minimize the impact race and ethnicity have on drug performance and don't proactively recruit representatives from historically marginalized groups.
Even as this issue gets more attention from the media and within the research world itself, most clinical trials still don't reflect the proportion of groups that have been historically marginalized in the U.S.
"We're not there yet," said Yvonne Maldonado, MD, a senior associate dean for faculty development and diversity at Stanford Medicine.
As an infectious disease expert who has worked with populations in sub-Saharan Africa, India and Latin America, Maldonado is among the foremost advocates for diversity and equity in clinical trials. She gives presentations on the topic and co-leads a Stanford Medicine community of researchers called Health Equity Action Leadership Network, which also includes a small pilot grant project geared toward helping study and educate academic leaders about health equity.
During the COVID-19 vaccine rollout, Maldonado was given an opportunity to walk the talk. Her pediatric vaccine trial turned out to be one of the highest enrolling—and most diverse—sites in the country.
She spoke about the state of diversity in clinical trials, the barriers to entry for potential participants, the opportunities for recruiters and why racial and ethnic diversity is so important in these studies.
What are the typical demographics of clinical trials?
When you look at the composition of the U.S., we're a minority-majority country, meaning there is not one group that represents more than half of all the U.S. population. Despite the rising number of individuals who come from racial and ethnic minority groups, we're not seeing that reflected in most clinical trials.
In 2015, Black and Latino people represented 30% of the U.S. population, but only 6% of participants in federally funded clinical trials and less than 5% of federally funded studies on lung disease included people of color. And as of 2011, 96% of genetic disease studies were conducted using European populations.
A big exception was COVID-19 vaccine trials. I helped make it clear by meeting with key stakeholders across the U.S. that we needed to see data on racial and ethnic minority populations. First, that would be a key driver in people's uptake of a vaccine. Because if people don't see themselves in these trials, they don't trust the data. And second, there are sometimes biological reasons why clinical trial outcomes might differ in some populations versus others.
We're just starting to plumb the depths of increasing diversity. But we're not there yet.
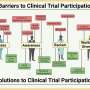
What have been some barriers to clinical trial participation for people of color?
I think we're doing a better job now. But there's also examples of people targeting people of color for a trial in which the participants didn't have informed consent, or the scientists weren't fully disclosing the risks and benefits of the trial. In some cases, there has been an over-representation of racial and ethnic minority populations.
Obviously the biggest example of that was the Tuskegee study in 1932 that looked at the natural history of syphilis among Black men. Some of those people were allowed to be experimented on to see what would happen to their disease over time without providing medical intervention, which is a hallmark classic case of racism in clinical trials. That type of situation clearly seeded mistrust in some would-be participants well into the future.
You can't promise a clinical trial will have a benefit for the participant. And if you have populations that are already living with limited resources, they may not trust the system to be fully honest with them. They may not have the desire to take more risks. There's also a lot of operational and day-to-day practical reasons why people might not want to get involved. The trial may take place during their work hours, they may not have transportation, the trial might be located a considerable distance from where they live, or they may not have a medical provider who can reflect with them on the study.
What are some of the concerns around trials that aren't diverse?
If the country is made up of a diverse population, then you're not getting a true picture of what a medical product might look like. If we're just looking at a homogeneous population in the study, that's not going to give you the full understanding of how drugs might work for certain populations. We need to understand the lens of biology and the basis of certain differences. For instance, there's a parallel of this in mice. Up until about 15 years ago, almost all U.S. experiments were done in male mice. When these drugs go to market, women experience adverse side effects twice as often as men. If we're not taking those into account in mice, we're going to miss them in humans as well.
How can we promote more diversity in clinical trials?
Currently, decisions to include more diversity rely on the researcher who gets funding. Every time you do a new study, you must re-engage communities, and you must have the funds to do that. Not every principal investigator is going to know how to do it in the best way. So, it's incumbent on government, academia and industry to build best practices along with community partners, knowing that there's not one formula for everyone.
When I did trials in Zimbabwe, for example, the first thing I did was engage the leadership of the Indigenous community. We helped the families understand why we wanted to conduct this study and why it might be helpful to their community and other communities like theirs. We also engaged in community townhalls to make sure everybody understood what the trial entailed so that there was a bidirectional flow of information. We hired all local Indigenous people to run the trials.
The key is to be willing to adapt your studies to suggestions that community members have, in terms of what might be more equitable for their community. For example, in our study, money wasn't well-received compensation. They wanted things like containers to store food or other practical items.
If you don't engage and gain trust with people, they're not going to understand why you're doing a trial, and this can lead to mistrust. Therefore, you really need to explain trials to people in a culturally resonant way, especially by engaging their respected community leaders and established practices.