This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Virtual drug quiets noise in heart tissue images
If you've ever tried taking a picture of a puppy, you likely ended up with a blur of fur. Now try reading a stock ticker on the puppy's fur, and you'll have the challenge that faces researchers studying electrical conduction of heart muscle.
The living heart contracts and relaxes every second as the heart pumps, but to researchers studying electrical conduction, this essential pumping is a confounding feature that they call "motion artifact." Researchers need to read that stock ticker—that is, the moving fluorescent signals that indicate the electrical functioning of heart cells—but to do so, they typically have to use drugs that stop the cells from beating while they take their measurements. This can affect cardiac electrophysiology and limit the ability to study how electrical conduction is coupled to both mechanical contraction and cellular structure.
Researchers at Washington University in St. Louis have developed a new computational approach to removing movement in images of expanding and contracting heart cells and tissues. By computationally removing movement, the algorithm mimics drug's action in stopping the heart, without compromising cellular structure or tissue contractility.
Results of the research, led by Nathaniel Huebsch, assistant professor of biomedical engineering, and Guy Genin, the Harold and Kathleen Faught Professor of Mechanical Engineering, both in the McKelvey School of Engineering, are published in the Proceedings of the National Academy of Sciences.
"When the heart beats, the electrical signal goes through the tissue, and that triggers mechanical squeezing," said Huebsch, who studies how mechanical cues affect heart development and disease.
"In many cases, deadly arrhythmias happen because the electric pulse becomes decoupled from the mechanical squeezing. Our group leverages biomaterials and genetics to study how inherited cardiomyopathies develop, and we are very excited to have a tool that allows us, for the first time, to directly and non-invasively study electro-mechanical coupling in cardiomyocytes," Huebsch continued.
The team's algorithm mimics the action of blebbistatin, a drug that inhibits motion of heart muscle but also can negatively impact the underlying structure of these cells. Genin and Huebsch worked with Eliot Elson, Emeritus Professor of Biochemistry and Molecular Biophysics at the School of Medicine on the method.
Development of the method and refinement for use in heart tissues engineered from human induced pluripotent stem cells (iPSCs) was co-led by Louis Woodhams, senior lecturer in mechanical engineering & materials science and a McKelvey Engineering alumnus who earned a master's and a doctorate in 2017 and 2022, and Jingxuan Guo, who earned a doctorate in mechanical engineering & materials science in 2022.
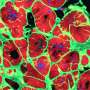
Virtual blebbistatin was created from two algorithms: one that has been successful in estimating displacements for cardiac mapping; and another that uses the first to map evolving signals back to a stabilized image of the tissue. Because virtual blebbistatin does not disrupt tissue contractility, Huebsch said this approach allowed the researchers to directly monitor the coupling of calcium waves, membrane voltage, and mechanical contraction beat by beat in his lab's iPSC heart tissue models, giving them an insight into diseases of the heart.
One of those diseases is hypertrophic cardiomyopathy, a genetic condition in which the heart muscle becomes thickened and stiff, making it more difficult for the heart to beat. The often-silent condition, found in about one in 500 people, is the most common natural cause of death in children and young adults, and often comes to light only after a young athlete dies suddenly during competition.
"Not everybody who has the gene mutation will develop the disease, which makes it really hard to manage the treatment of the patients," Huebsch said. "Normally, if someone is at high risk to develop deadly arrhythmias, you'd want to implant a pacemaker in them. But since not everyone with these mutations will develop the disease, that's not a good option. On top of this challenge, studies on new drugs to treat the disease require treatment to begin very early on in the course of disease in controlled animal models."
The team tested virtual blebbistatin on cardiomyocytes derived from human iPSCs and engineered heart muscle as a model. The first engineered heart tissues were developed in Elson's lab in the 1990s. The tissue models from Huebsch's lab extend these using patient-specific heart cells that can be made from a specific patient's fat cells using dynamic and elastic environments that replicate disease and development. and using the latest genetic tools to understand how genes combine with epigenetic factors to start determining which patients are in danger of developing severe heart disease.
In the study, they compared engineered heart muscle to heart cells that were given a genetic mutation for hypertrophic cardiomyopathy, and were able to compare mechanical-electrical coupling in the two by removing about 95% of motion from them.
"The electrical-mechanical coupling of heart tissue is a key factor in the mechanobiology of heart function," Genin said. "This combination of the Huebsch lab's advances in laboratory disease modeling with the latest solid mechanics tools provides much potential for understanding how epigenetic factors affect the mechanobiology of disease progression."
More information: Woodhams, Louis G. et al, Virtual blebbistatin: A robust and rapid software approach to motion artifact removal in optical mapping of cardiomyocytes, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2212949120
Louis G. Woodhams et al, Virtual Blebbistatin image stabilization software, Zenodo (2023). DOI: 10.5281/zenodo.7897265