This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Advanced imaging reveals altered brain chemistry of babies with congenital heart disease
Researchers at Children's National Hospital used magnetic resonance spectroscopy to find new biomarkers that reveal how congenital heart disease (CHD) changes an unborn baby's brain chemistry, providing early clues that could someday guide treatment decisions for babies facing lifelong health challenges.
Published in the Journal of the American College of Cardiology, the findings detail the ways that heart defects disrupt metabolic processes in the developing brain, especially during the third trimester of pregnancy when babies grow exponentially.
"Over the past decade, our team has been at the forefront of developing safe and sophisticated ways to measure and monitor fetal brain health in the womb," said Catherine Limperopoulos, Ph.D., director of the Center for Prenatal, Neonatal and Maternal Health Research at Children's National. "By tapping into the power of advanced imaging, we were able to measure certain maturational components of the brain to find early biomarkers for newborns who are going to struggle immediately after birth."
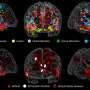
In one of the largest cohorts of CHD patients assembled to date, researchers at Children's National studied the developing brains of 221 healthy unborn babies and 112 with CHD using magnetic resonance spectroscopy, a noninvasive diagnostic test that can examine chemical changes in the brain. They found:
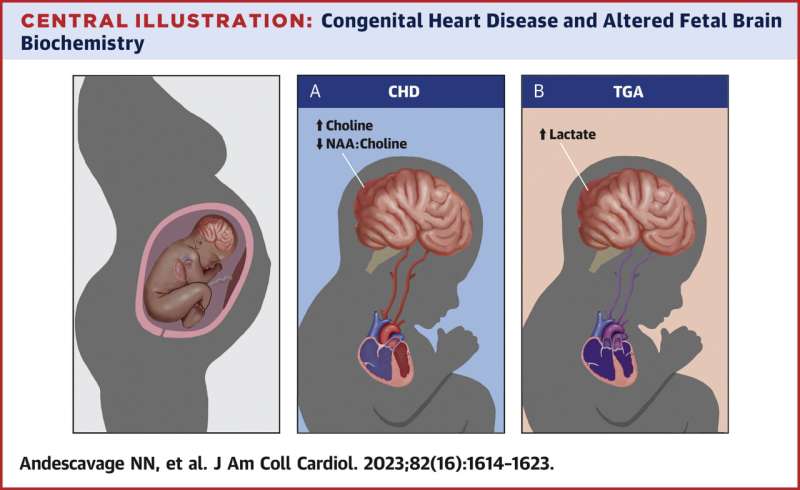
- Those with CHD had higher levels of choline and lower levels of N-Acetyl aspartate-to-choline ratios compared to healthy babies, potentially representing disrupted brain development.
- Babies with more complex CHD also had higher levels of cerebral lactate compared to babies with two ventricle CHD. Lactate, in particular, is a worrying signal of oxygen deprivation.
Specifically, elevated lactate levels were notably increased in babies with two types of heart defects: transposition of the great arteries, a birth defect in which the two main arteries carrying blood from the heart are switched in position, and single ventricle CHD, a birth defect causing one chamber to be smaller, underdeveloped or missing a valve.
These critical heart defects generally require babies to undergo heart surgery not long after birth. The elevated lactate levels also were associated with an increased risk of death, highlighting the urgency needed for timely and effective interventions.
The research suggests that this type of imaging can provide a roadmap for further investigation and hope that medicine will someday be able to better plan for the care of these children immediately after their delivery. "With important clues about how a fetus is growing and developing, we can provide better care to help these children not only survive, but thrive, in the newborn period and beyond," said Nickie Andescavage, M.D., Children's National neonatologist and first author on the paper.
CHD is the most common birth defect in the United States, affecting about 1% of all children born or roughly 40,000 babies each year. While these defects can be fatal, babies who survive are known to be at significantly higher risk of lifelong neurological deficits, including lower cognitive function, poor social interaction, inattention and impulsivity. The impact can also be felt in other organ systems because their hearts did not pump blood efficiently to support development.
Yet researchers are only beginning to pinpoint the biomarkers that can provide information about which babies are going to struggle most and require higher levels of care. The National Institutes of Health (NIH) and the District of Columbia Intellectual and Developmental Disabilities Research Center supported the research at Children's National to improve this understanding.
"For many years we have known that the brains of children with severe heart problems do not always develop normally, but new research shows that abnormal function occurs already in the fetus," said Kathleen N. Fenton, M.D., M.S., chief of the Advanced Technologies and Surgery Branch in the Division of Cardiovascular Sciences at the National Heart, Lung and Blood Institute.
"Understanding how the development and function of the brain is already different before a baby with a heart defect is born will help us to intervene with personal treatment as early as possible, perhaps even prenatally, and improve outcomes."
More information: Nickie N. Andescavage et al, Magnetic Resonance Spectroscopy of Brain Metabolism in Fetuses With Congenital Heart Disease, Journal of the American College of Cardiology (2023). DOI: 10.1016/j.jacc.2023.08.013