This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cellular atlas of amygdala reveals new treatment target for cocaine addiction
Researchers at University of California San Diego School of Medicine and the Salk Institute for Biological Studies have created a unique, cell-by-cell atlas of the amygdala, a small structure deep within the brain that plays a crucial role in controlling emotional responses to drugs. The findings, published in Nature Neuroscience, helped the researchers identify a potential new treatment for cocaine addiction, a disease that is poorly understood at the molecular level and has virtually no approved pharmacological treatments.
"There are some drugs that can help treat other addictions, such as those to opioids or nicotine, but there are currently no safe and effective drugs approved for cocaine addictions," said co-senior author Francesca Telese, Ph.D., an associate professor in the Department of Psychiatry at UC San Diego School of Medicine. "These findings help address that problem and could also point to universal molecular mechanisms of addiction that we haven't understood until now."
Cocaine is a widely used illicit drug and addiction to cocaine is a major public health concern, associated with a rising number of overdose deaths and a high rate of relapse. Despite the threat cocaine addiction poses, not every person who uses cocaine develops an addiction. According to the National Institute on Drug Abuse, an estimated 4.8 million people used cocaine in 2021, while only 1.4 million people had a cocaine use disorder.
"Some people use cocaine recreationally and never develop an addiction, while others are extremely susceptible to addiction after very little exposure to the drug or may relapse even after years of abstinence," said Telese "Our long-term goal is to understand why there are these inter-individual differences in drug addiction behavior."
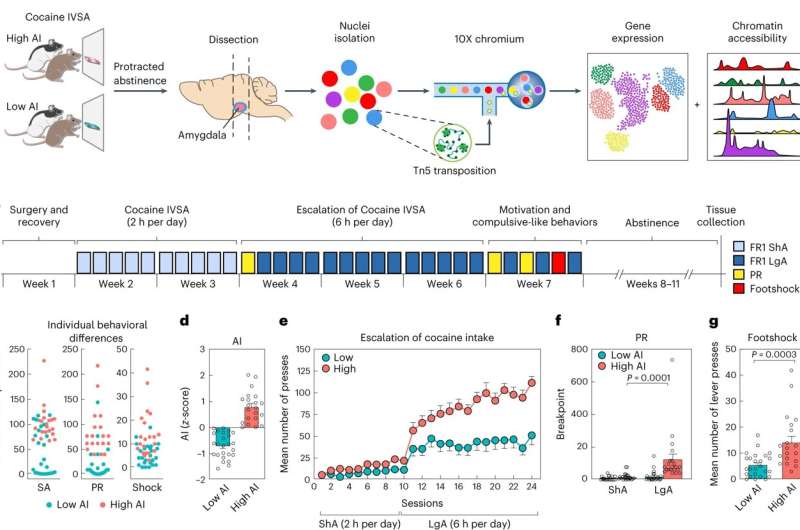
The researchers studied brain samples from rats that had been allowed to self-administer cocaine for an extended period before being cut off from the drug for a period of abstinence. These samples were obtained from the cocaine brain bank at UC San Diego, established by study co-authors Abraham A. Palmer, Ph.D., and Olivier George, Ph.D., both professors in the Department of Psychiatry at UC San Diego School of Medicine.
"The cocaine brain bank is an exceptional resource and was invaluable for this study because it allowed us to study a cohort of rats with a large amount of genetic variety, which mimics the diversity we see in human populations," said Telese. "Further, using a model of cocaine addiction where rats administered the drug to themselves let us look at the connection between our molecular discoveries and actual addiction behaviors."
The team used single-cell sequencing to determine what genes were expressed in individual cells from the rats' amygdala, a part of the brain that is central to processing emotions and is highly active in people with addictions.
"Being able to look at individual amygdala cells from rats with different vulnerabilities to addiction was an asset for our study because we wanted to understand how specific cell populations of the amygdala contribute to addiction development," Telese added.
To make sense of the large amount of data generated through their sequencing experiments, Telese collaborated closely with bioinformatics expert and co-senior author Graham McVicker, Ph.D., an associate professor at the Salk Institute of Biological Studies and an assistant adjunct professor in the Department of Cellular & Molecular Medicine at UC San Diego School of Medicine. Jess Zhou, a UC San Diego graduate student working with McVicker, developed the bioinformatics workflow needed to assemble their sequencing data into a molecular atlas of the rat amygdala.
The results revealed never-before-seen connections between addiction behaviors and genes involved in energy metabolism.
"This tells us that energy metabolism may be playing a key role in the activity of neurons in the amygdala and that this effect could be contributing to addiction-like behaviors," said Telese. "This is a brand-new way of thinking about the molecular biology of cocaine addiction."
In addition to identifying molecular factors that influence cocaine addiction behaviors, the researchers were able to test a drug in the rats that helped reverse these behaviors by targeting an enzyme involved in both energy metabolism and signaling between neurons.
"The fact that we were able to link our findings at the cellular level to behaviors exhibited in the rats and were even able to modify these behaviors with a drug puts us one step closer to understanding the extremely complex mechanisms in the brain driving addiction and relapse," added Telese.
The researchers are now working on larger sample-size studies that can help determine how much of the effects they observed were based on preexisting genetics in the rats and how much were based on responses to extensive cocaine usage.
"This research suggests that preexisting genetics may play a much bigger role in addiction than we've previously understood," said Telese. "Unraveling these genetics will be key to improving personalized medicine for addictions. If we don't understand the risk of relapse in individual people, we can't fully understand the disease."
More information: Zhou, J.L. et al. Single-nucleus genomics in outbred rats with divergent cocaine addiction-like behaviors reveals changes in gene amygdala GABAergic inhibition. Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01452-y www.nature.com/articles/s41593-023-01452-y