This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel dynamic imaging technology captures the body's immune response to COVID-19 infection
A team of UC Davis scientists has used dynamic total-body positron emission tomography (PET) to provide the first imaging of the human body's immune response to COVID-19 infection in recovering patients. Their work, published in Science Advances, could lead to a better understanding of how the body's immune system responds to viral infections and develops long-term protection against re-infection.
The researchers used the uEXPLORER total-body PET scanner. It is an innovative imaging technology developed at UC Davis in collaboration with United Imaging Healthcare.
Dynamic PET involves injecting very small amounts of radiotracers in the patient, followed by continuous imaging over a period of time. It essentially creates a movie showing the radiotracer kinetics (distribution over time) within the body. Mathematical models can then be applied to extract biologically-relevant information.
Total-body PET scanners enable simultaneous dynamic imaging and kinetic modeling in all body organs. They have significantly higher sensitivity than conventional PET systems. This translates into better image quality while enabling the use of lower radiotracer injection doses.
"Dynamic total-body PET is currently the only available technology with acceptable radiation dose that allows noninvasive quantitative measurements of immune cell distribution and trafficking (movement) inside all tissues in living humans," said first author Negar Omidvari, assistant project scientist in the UC Davis Department of Biomedical Engineering.
Noninvasive technology to study the body's immune response
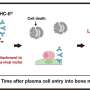
The study presents the first use of dynamic PET and kinetic modeling to measure CD8+ T cell distribution in humans. CD8+ T cells are specific immune cells with CD8 protein on their surface. During a viral infection, naïve (non-customized) CD8+ T cells become activated and cytotoxic. This means they find and kill infected cells. Some of these CD8+ cells develop into antigen-specific memory T cells for long-term protective memory against reinfection.
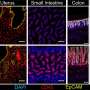
These cells circulate in the blood but mostly reside and function in non-blood tissues, particularly the lymphoid organs, such as the bone marrow, spleen, tonsils and lymph nodes.
"There has been a growing interest in studying the critical role of CD8+ T cells in immune response and memory. However, evaluating immunological changes in non-blood tissue is challenging due to the invasive nature of biopsies. In some cases, it is not even practical in certain anatomical regions of living participants, such as the brain, spinal cord, cardiopulmonary tissue and vascular tissue," Omidvari said. "So, the challenge was to find noninvasive quantitative methods for measuring CD8+ T cell distribution and trafficking in the body that are safe to also use in healthy people."
For the study, the researchers enrolled three healthy individuals and five patients recovering from COVID-19 infection. The recovering patients had mild or moderate symptoms and were not hospitalized.
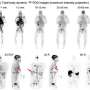
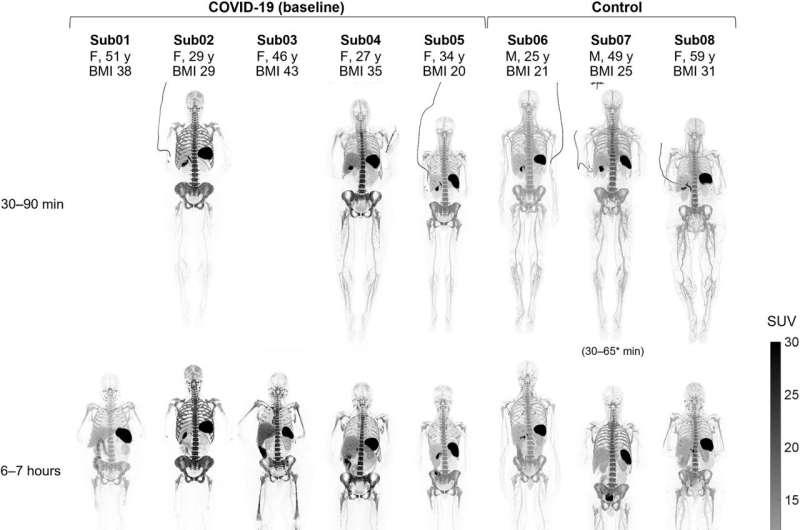
The team injected the participants with a small amount of a radioactive liquid that includes an immunoPET radiotracer (89Zr-Df-Crefmirlimab) that targets human CD8. For each participant, the team took a dynamic 90-minute scan, a 60-minute scan at six hours, and a 60-minute scan at 48 hours after the injection. The recovering patients also did the same set of scans four months later.
The researchers measured the activity of the radiotracer in the blood and non-blood tissue on PET images. They did kinetic modeling to separate the effect of blood circulation on tissue. This allowed them to measure the radiotracer uptake in tissues independent of when the imaging was done and differences in the blood of each participant.
Main findings
With the total-body PET, the researchers could do a noninvasive measurement of the T cell distribution with remarkable image quality throughout the whole body, in all tissue types. Their study showed a high uptake of CD8+ T cells in the lymphoid organs of all participants. The highest uptake was in the spleen, followed by the bone marrow, liver, tonsils and lymph nodes.
The most significant finding was the increased concentrations of CD8+ T cells in the bone marrow of recovering COVID patients compared to the healthy controls. In the follow-up imaging (acquired at 6 months post infection), these concentrations in recovering patients were slightly higher than those acquired at around 2 months post infection (baseline) in all bone marrow regions.
"Bone marrow has been identified as a major pool and the preferred site for proliferation of memory CD8+ T cells following a viral infection. This trafficking of memory T cells to certain tissues like the bone marrow is critical to developing immune memory after viral infection," Omidvari explained.
Less radiation, better results
The specificity of the immunoPET tracer combined with the high detection sensitivity of total-body PET provides a new platform for scientists to study the immune response and memory in all organs over the long term in a non-invasive way.
"What is fundamentally important about this study is that it showed the potential of total-body PET to assess T-cell distributions across the entire human body, with the image quality necessary for detailed modeling, and with a radiation dose that is low enough to allow its broad application to study the immune response in humans," said Simon R. Cherry, a UC Davis physicist and distinguished professor emeritus of biomedical engineering and radiology.
"In our case, we were able to characterize the dynamics of this immunoPET tracer in healthy control subjects and in patients with infectious disease (COVID-19), which is an important first," Cherry added.
The team pointed to many potential applications for this method. It could be used to study the immune response during viral infection, immune memory after viral infection, and treatment response evaluation in cancer patients. It might also extend to studies of infectious disease, autoimmune disease and transplant, and can be used for prognosis as well as therapeutic and vaccine developments.
More information: Negar Omidvari et al, First-in-human immunoPET imaging of COVID-19 convalescent patients using dynamic total-body PET and a CD8-targeted minibody, Science Advances (2023). DOI: 10.1126/sciadv.adh7968