October 31, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Can female fertility survive harsh cancer therapy? Scientists who turned to animal models say the answer is 'yes'
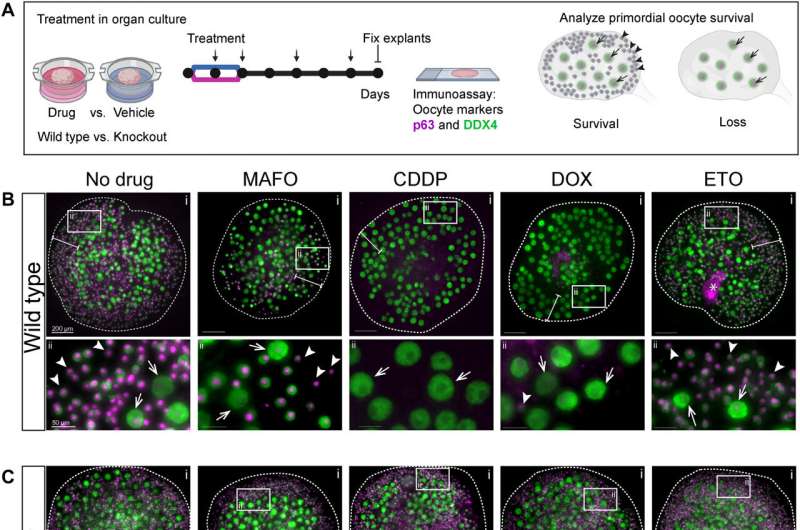
Cancer treatment can rob childbearing-age women of fertility, but new research has uncloaked how the body's own traitor protein conspires with chemo and other harsh therapies against the ovaries' primordial follicles, home of immature oocytes—the entire ovarian egg reserve.
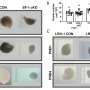
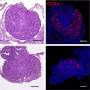
A fertility-damaging protein called CHEK2, when triggered by chemo's destruction of DNA, is singlehandedly to blame for coordinating deletion of primordial follicles containing immature eggs—oocytes—according to a small research group at the Jackson Laboratory in Maine. But in a stunning discovery, albeit in mouse models conducted as part of the research, the team found that blocking CHEK2 with an inhibitor stops the protein's follicle-destroying activity, preserving the vital ovarian egg reserve and fertility.
Jackson Lab scientists say CHEK2 is an attractive target for future fertility-preserving interventions that ensure reproductive health and the likelihood of a successful pregnancy for women cancer survivors. When CHEK2 is deficient, these scientists say, oocytes can survive chemotherapy.
"Women are born with a finite supply of primordial follicles that constitute the ovarian reserve," writes Chihiro Emori, lead author of a new study published in the journal Science Advances.
"Hormonal sufficiency and optimal female health depend on a robust supply of follicles in the ovary. This is because developing follicles are a major source of female hormones, such as estrogens that are responsible for the development and regulation of the female reproductive system and overall reproductive health."
Cryopreservation of eggs is the way many women of childbearing age have maintained the hope of having children years after cancer therapy followed by in vitro fertilization, which may or may not be successful. The new research is pointing the way toward preserving fertility altogether. With the aid of mouse models, Jackson Lab researchers have now defined why fertility is lost, and they're proposing CHEK2 inhibition as a way to preserve a robust egg supply.
Emori is a postdoctoral researcher in the laboratory of Dr. Ewelina Bolcun-Filas, who was part of this study, and has long been a champion of fertility preservation research. Emori and Bolcun-Filas were aided in the new analysis by researcher Zachary Boucher. The team examined mechanisms underlying fertility lost to cancer therapy, and their latest study shines a light on a once obscure protein whose full name is Checkpoint kinase 2, but more frequently is identified by its shorthand moniker, CHEK2.
CHEK2 has a first cousin called CHEK1, which is activated during cancer therapy when the DNA double helix is damaged and undergoes a single-strand break in the helix. DNA repair is possible after a single-strand break. CHEK2 is activated in double-strand helical breaks, the most lethal type of DNA damage, the Jackson Lab scientists explain in their research.
Once activated, CHEK2 triggers two downstream targets—Tap63 and p53—that direct the elimination of oocytes. The team additionally discovered that TAp63 and p53 can also be triggered by a class of chemotherapy medications called alkylating agents, which contribute to DNA damage and oocyte elimination. As cancer medications, alkylating agents impede DNA replication and blunt cancer cell growth, but accelerate problems relative to ovarian health by exacerbating primordial follicle damage, and hence the loss of fertility.
"Genotoxic cancer treatments kill cancer cells by inducing DNA damage in the form of single-strand breaks and double-strand breaks, which are more detrimental to fast-dividing cancer cells than healthy cells. However, these treatments can also damage healthy cells including oocytes," according Emori and colleagues. "DNA damage inflicted in primary oocytes residing in primordial follicles is the major trigger of radiation- and chemotherapy-induced primordial follicle elimination."
When women of childbearing age undergo cancer treatment and lose primordial follicles as a result of that therapy, their ovaries cease producing vital female hormones, which triggers premature menopause. Once in menopause, the risk rises for heart disease, stroke and cognitive decline.
"How different chemotherapy drugs inflict damage in ovaries and in meiotically arrested oocytes specifically, and how this damage triggers primordial oocyte elimination is still not fully understood," Emori and colleagues report.
"Chemotherapy drugs can damage DNA both directly and indirectly by increasing oxidative stress," the researchers add, noting "it is possible that these drugs induce indirect DNA damage in oocytes as a result of elevated reactive oxygen species that accumulate over time. Co-administration of drugs reducing oxidative stress has been shown to decrease their ovotoxicity."
While a segment of the research spelled out how deleterious oocyte damage inflicted by the activation of CHEK2 can be, Emori and colleagues took the research a major step forward and asked this tantalizing question: What if CHEK2 is inhibited? To find out, they turned to an investigational drug known as AZD7762.
"AZD7762 blocked activation of CHEK2 during drug treatment and improved oocyte survival. This suggests that inhibition of the CHEK2 pathway resulted in sufficient DNA repair to evade apoptosis even after inhibitor withdrawal," the researchers assert.
"Evidence from prior work and other studies indicate that primordial oocytes that survive genotoxic insults do indeed support normal ovarian function and fertility. Moreover, genetic inactivation of CHEK2 almost completely protected primordial oocytes," the team concludes.
More information: Chihiro Emori et al, CHEK2 signaling is the key regulator of oocyte survival after chemotherapy, Science Advances (2023). DOI: 10.1126/sciadv.adg0898
© 2023 Science X Network