This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Jet lag disorder associated with shift work can lead to brain changes increasing appetite
Scientists have uncovered why night shift work is associated with changes in appetite in a new University of Bristol-led study. The findings, published in Communications Biology, could help the millions of people that work through the night and struggle with weight gain.
Scientists from Bristol and the University of Occupational and Environmental Health in Japan, sought to understand how "circadian misalignment"—a phenomenon commonly associated with "jet-lag" whereby the body's biological clock is disrupted—affects the hormones responsible for regulating appetite.
Prevalent in night shift workers, in this new study, the international team reveal how circadian misalignment can profoundly alter the brain's regulation of hormones controlling hunger to the detriment of metabolic health.
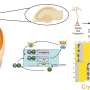
The team focused on glucocorticoid hormones in the adrenal gland which regulate many physiological functions including metabolism and appetite. Glucocorticoids are known to directly regulate a group of brain peptides controlling appetitive behavior, with some increasing appetite (orexigenic) and some decreasing appetite (anorexigenic).
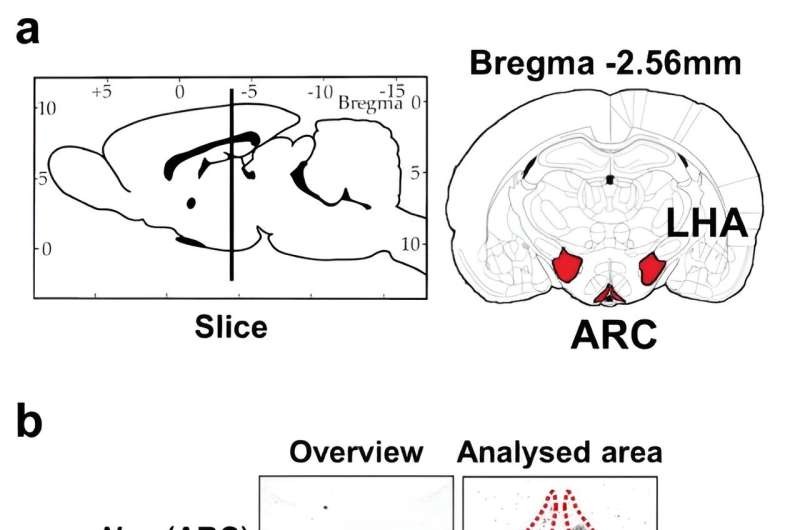
In an experiment using animal models, comprising a control group and a out-of-phase "jet-lagged" group, the team found misalignment between light and dark cues led the out-of-phase group's orexigenic hypothalamic neuropeptides (NPY) to become dysregulated, driving an increased desire to eat significantly more during the inactive phase of the day.
Strikingly, the team discovered that rats in the control group ate 88.4% of their daily intake during their active phase, and only 11.6% during their inactive phase. In contrast, the "jet-lagged" group consumed 53.8% of their daily calories during their inactive phase (without an increase in activity during this time). This equated to nearly five-times more (460% more) than what the control group consumed during the inactive phase. These results show that it is timing of consumption that has been affected.
This new discovery revealed how completely, and significantly, disordered the neuropeptides become when daily glucocorticoid levels are out of synch with light and dark cues. However, the authors suggest the neuropeptides identified in this study may be promising targets for drug treatments adapted to treat eating disorders and obesity.
Dr. Becky Conway-Campbell, research fellow in Bristol Medical School, Translational Health Sciences (THS) and the study's senior author, said, "For people working throughout the night, a reversed body clock can play havoc with their health.
"For those who are working night shifts long-term, we recommend they try to maintain daylight exposure, cardiovascular exercise and mealtimes at regulated hours. However, internal brain messages to drive increased appetite are difficult to override with 'discipline' or 'routine' so we are currently designing studies to assess rescue strategies and pharmacological intervention drugs. We hope our findings also provide new insight into how chronic stress and sleep disruption leads to caloric overconsumption."
Stafford Lightman, Professor of Medicine at Bristol Medical School, THS and co-senior author on the study, added, "The adrenal hormone corticosterone, which is normally secreted in a circadian manner, is a major factor in the daily control of brain peptides that regulate appetite. Furthermore when we disturb the normal relationship of corticosterone with the day to night light cycle it results in abnormal gene regulation and appetite during the period of time that the animals normally sleep.
"Our study shows that when we disturb our normal bodily rhythms this in turn disrupts normal appetite regulation in a way that is at least in part a result of desynchrony between adrenal steroid hormone production and the timing of the light and dark cycle."
Dr. Benjamin Flynn, one of the study's co-authors who conducted the study while at Bristol but is now based at the University of Bath, added, "This is further evidence of how phase shift 'jet-lag' affects feeding behaviors and neuronal gene expression—data important for shift work co-morbidity research."
More information: Mitsuhiro Yoshimura et al, Phase-shifting the circadian glucocorticoid profile induces disordered feeding behaviour by dysregulating hypothalamic neuropeptide gene expression, Communications Biology (2023). DOI: 10.1038/s42003-023-05347-3