This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research reveals previously unknown qualities of a gene vital to a cell's mitochondrial structure and function
A key takeaway from first-year biology is that mitochondria are the powerhouses of cells—it's the thing most people know about them. However, mitochondria perform a large array of functions for cells beyond generating the chemical energy that powers a cell's biochemical reactions. They play a role calcium signaling and storage, signaling between cells and cell death. And through these various and vital mitochondrial functions, a master regulator is the OPA1 gene.
For a long time, researchers have known that OPA1 plays a crucial role in mitochondria. For example, OPA1 helps maintain the architecture of the mitochondria's inner membrane. Without that maintenance, a protein, cytochrome c, can leak into the cell and trigger cell death at the wrong time.
While researchers have long known that OPA1 is vital to mitochondria and mitochondrial membranes in human cells, not much has been known about how OPA1 does its work. But research recently published in the journal Nature sheds new light on how OPA1 helps reshape mitochondrial membranes and how that translates to cellular health.
"We've known for a long time that this gene exists, we know that it's important in a variety of diseases, including cardiovascular disease, cancer, neurodegenerative disease," says principal investigator Halil Aydin, a University of Colorado Boulder assistant professor of biochemistry. "What we didn't know is how it functions. Our goal is to understand how it works and then in the future use that as a blueprint for developing therapeutic strategies or drugs."
Unexpected plasticity
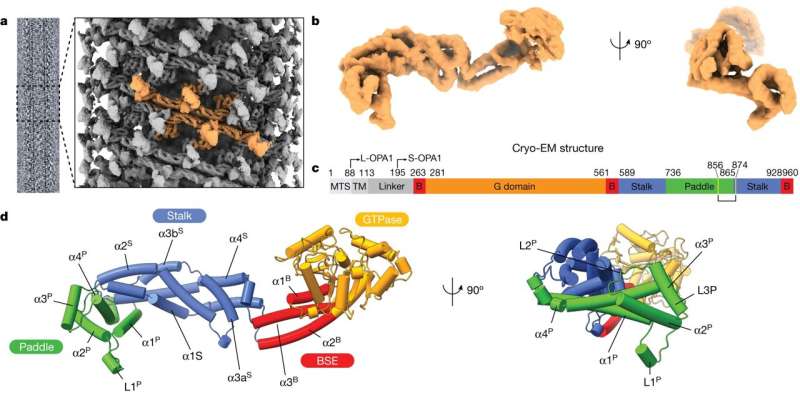
Aydin and his research group first separated OPA1 from cells and studied it in vitro to understand its atomic structure, which was no easy feat. When OPA1 is remodeling mitochondrial membranes in the cell, that process happens extremely fast, "we're talking at the microsecond level," Aydin says. "This was always confusing to me as a biologist, how it can happen so fast."
One of the research group's biggest discoveries in studying OPA1 outside the cell was its plasticity and flexibility. Aydin says he had not been expecting to see that degree of plasticity, and while it went a long way toward explaining how OPA1 is so dynamic, it makes getting a clear photo very difficult.
The researchers used an electron microscope to study OPA1, "but imagine taking a picture with your phone," Aydin says. "You move it even a little, something in the background moves even a tiny bit, and the picture is blurry. So, it was tough for us to find the conditions where we could just lock in on OPA1 and see it clearly. We were shocked by how dynamic and flexible it is."
With a better understanding of OPA1's atomic structure, Aydin and his research colleagues were better able to study it at work in mitochondrial membranes. They saw that through a particular lipid-binding process in mitochondrial membranes, OPA1 can help shape and reshape the mitochondria.
"Mitochondria are shaped like a tubule," Aydin says. "If you think about them like a balloon animal, OPA1 changes the chape of the balloon depending on cellular needs or energy demands. OPA1 is like the person making the balloon animals."
Aydin and his research group are currently collaborating with researchers at Vanderbilt University to directly study OPA1 activity in stem cell-derived neurons. Via molecular manipulation, they are studying how changes in OPA1's activity affect essential cellular functions.
A clearer path
A broader aim of the newly published and ongoing research is to understand how molecular observations in the lab translate to cellular health, Aydin says. There are more than 400 disease variants that affect the OPA1 protein, and by determining its molecular architecture "we can better map all these disease mutations and understand how they affect protein functions," Aydin says. "In the case of the neurons, that can lead to a decrease in neuronal health and cause optic neuropathies and other neurodegenerative disorders."
For example, autosomal dominant optic atrophy, a hereditary disorder that can lead to progressive loss of vision or childhood blindness, is caused by mutations in the OPA1 gene. A goal of gene therapies in the future will be targeting and correcting this mutation, Aydin says.
"In science, we call this the bottom-up approach," he says. "We start by looking at a single molecule, then the organelle, then the cell, then tissue, then systems. The power of this is that once we understand something at the atomic level, that translation gets clearer at each step that follows. So, as we learn more about how OPA1 functions, the path to better drugs and therapeutics becomes clearer."
More information: Alexander von der Malsburg et al, Structural mechanism of mitochondrial membrane remodelling by human OPA1, Nature (2023). DOI: 10.1038/s41586-023-06441-6