This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers uncover mechanism that links NAD+ to fertility problems
A woman's fertility normally decreases by her late 30s with reproductive function eventually ceasing at menopause. It is known that a small molecule called nicotinamide adenine dinucleotide (NAD+) plays a critical role in this decline, and Buck scientists have revealed how this happens and have identified potential new approaches to enhance reproductive longevity.
"Studying ovarian biology and reproductive aging is not just about trying to increase fertility, but really about the overall health of females," said Buck President and Chief Executive Officer Eric Verdin, MD, senior author of the study, which is in iScience. "We want to understand the processes that lead to decreased fertility that are linked to menopause and therefore linked to overall lifespan and healthspan of women. This is a perspective shift that needs to happen."
"We really made a step forward in understanding the role of NAD in ovarian function and how a female's reproductive lifespan progresses," said Rosalba Perrone, Ph.D., co-first author of the study and a senior research scientist in the Verdin lab. "What makes this research exciting is demonstrating we can modulate NAD+ to affect fertility."
NAD+, which is present in all cells throughout the human body, begins to decline with age and maintaining optimal levels is vital for key cellular functions and healthy aging, said Perrone. Recently, it became clear that the same decline was occurring in the ovaries, contributing to the natural winnowing over time of egg numbers combined with reduced egg quality, both of which contribute to decreased fertility in females.
"So we knew that NAD was really important in promoting ovarian function, but what we didn't know is why it declines in the first place, what is the driver of this decline?" she said.
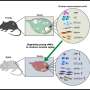
To uncover the molecular mechanisms regulating ovarian NAD+ loss, first the researchers began by adding another piece of the puzzle: CD38, an enzyme known to be one of the main culprits in degrading NAD+.
What happens during aging, Perrone explained, is that more CD38 is expressed, leading to more degradation of NAD+, which accelerates the aging processes. However, CD38's role in female reproductive function was not established. "We realized nobody had even looked at where this protein was located in the ovaries, so we really started from scratch," she said.
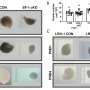
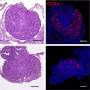
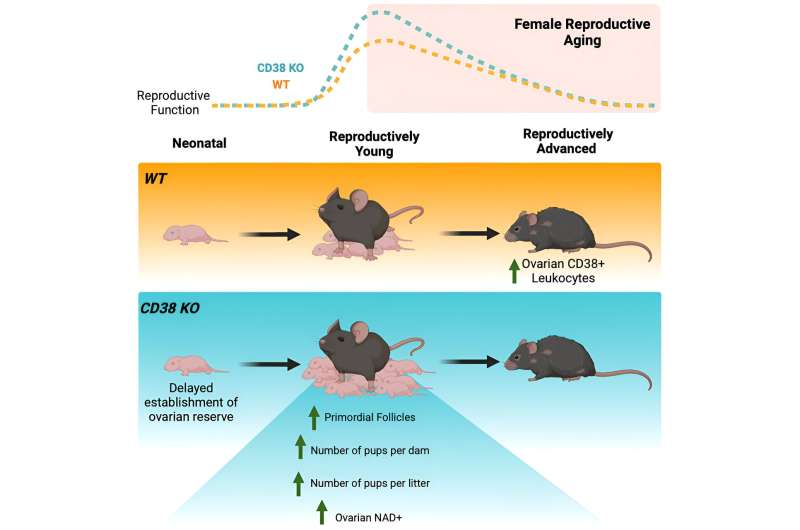
The team found that CD38 was primarily found in ovarian immune cells and located in specific structures outside the ovarian follicles.
"We also saw that CD38 increases with age within the ovaries, which is the first time this has been shown," she said, noting that the more CD38 was present, the less NAD+ there was. "Discovering the missing link in the literature, showing that CD38 expression led to NAD+ decline, was the most exciting part."
Additionally, the team investigated a type of mouse that completely lacks CD38 and found that mice without CD38 have more "primordial follicles" which eventually mature to release an egg. Females are born with a finite number of such follicles which determine the length of the ovarian lifespan, and therefore the fertility of an individual. "We were amazed to see that the increased number of primordial follicles was associated with more offspring, reinforcing the biological relevance of our observations," she said.
Showing that CD38 regulates ovarian function and fertility through NAD+ metabolism means that targeting this molecule may offer new approaches to enhance reproductive longevity.
"CD38 absolutely is a druggable target," said Perrone, noting that there are a number of small molecules and antibodies already available that specifically recognize CD38's ability to degrade NAD+. "What made this research rewarding is knowing that we can potentially modulate how CD38 consumes NAD+ and increase fertility when conception is desired."
While this work builds a comprehensive picture of CD38's role in consuming NAD+ and affecting fertility, Perrone emphasizes that this work reaches far beyond fertility.
"We know that menopause, which we commonly associate with loss of reproductive potential, is actually linked to overall lifespan and healthspan," she said. "So understanding what makes our reproductive organs work better is really important to study the aging process in general, and in particular for women."
More information: Rosalba Perrone et al, CD38 regulates ovarian function and fecundity via NAD+ metabolism, iScience (2023). DOI: 10.1016/j.isci.2023.107949